Article Text
Statistics from Altmetric.com
Introduction
Maintaining safety in a haemodialysis (HD) unit is paramount.1 Low-molecular-weight heparin (LMWH) is commonly used as an anticoagulant to prevent clot forming in the extracorporeal circuits (ECCs).2 3 Incorrect dosages of anticoagulant may bring risks to patients with HD. Presently, most HD units determine their own dosages of LMWH according to their own experience, and guided by clinical efficacy.4 Monitoring of low-range activated clotting time (ACT-LR) and anti-Xa activity is rarely undertaken or available. Chinese national standard operational procedure (SOP) for HD has suggested no specific LMWH regimen, leaving it to the discretion of individual HD units.5 Most Chinese dialysis units appear to have an array of heparin regimens of their own.6
Our HD unit has been built within a public comprehensive hospital in the south of China and was awarded Australian Council of Health and Safety accreditation.7 It has 50 dialysis stations where 200 patients with chronic HD are dialysed in 3 shifts. Typically, it gives a single bolus intravenous injection of dalteparin at 60 IU per kg body weight before dialysis.5 The measurement of ACT-LR or anti-Xa activity is not available in our hospital. The bolus dosage would simply be increased by 250 IU if there were signs of clot, or decreased by 250 IU if bleeding was prolonging.
In the present incident, 12 patients with HD developed clot in their ECCs almost simultaneously about an hour into dialysis. The ECCs were replaced swiftly so that all the dialysis treatments were completed as scheduled and the patients were safe. The hospital’s incident management team (IMT) was notified immediately. A dalteparin admixture error was suspected. Quality improvement (QI) meetings were held and team members brainstormed for possible root causes and identified possible solutions.
Methods
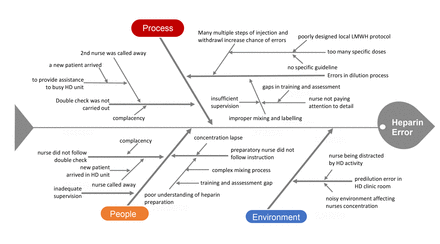
Following on the heels of the incident, a QI meeting was held and members of IMT, Pharmacy Department, Clinical Service Department and Nursing Department were invited to participate. The routine dalteparin process from prescription to admixture, check and final administration was reconstructed and scrutinised item by item by all the participants with fishbone diagram and 5-whys as the guide. The fishbone diagram in figure 1 lists our deficiencies in terms of people, process and environment as we found them. The 5-whys technique drives us to keep asking why. Ideas generated by both tools helped immeasurably our QI team in its search for root causes of the dalteparin medication errors and their possible resolutions in its brainstorming meetings.
Fishbone diagram. HD, haemodialysis; LMWH, low-molecular-weight heparin.
Results
In the analysis of the incident, improper admixture of dalteparin–saline was found to be the cause. Heparin-coated circuits are not available in China and most of our patients are provided with polysulfone membranes. It was revealed that as many as 26 different patient-specific dalteparin dosages were being used in the 200 patients with chronic HD. At the time, the dalteparin predilution was prepared in the HD unit clinic room by two nurses following double check. In making the dalteparin admixture in question, each dilution bag was to contain dalteparin 50 000 IU/100 mL of 0.9% saline. Each bag should be prepared using 10×0.5 mL ampoules of dalteparin 5000 IU/0.5 mL, so 10×5000 IU=50 000 IU. In this incident, 1 of the 2 preparatory nurses who was newly qualified made an error by putting twice the amount of 10×0.5 mL ampoules of dalteparin 5000 IU/0.5 mL, so 20×5000 IU=1 00 000 IU into 1 dilution bag and none in the other. A drug label containing the name of the drug and the dose (dalteparin 10×0.5 mL of 5000 IU/0.5 mL=50 000 IU) should have been placed on the dilution bags after admixture. In the present case, the preparatory nurse by mistake had mislabelled the bag. Double check failed because the second nurse on duty was away to attend to a new patient that arrived at the HD unit. The admixture without dalteparin was withdrawn from the dilution bag manually and given to the 12 patients under discussion. They went on and developed clot in their ECCs because they had been given the ‘fake’ dalteparin improperly prepared as described. Luckily, none of the patients given the ‘double’ dalteparin suffered any significant bleeding.
During the subsequent QI meetings, it was agreed that there were too many specific dalteparin dosages prepared and used and too much discretion allowed for their use. In the new regimen as decided in these meetings, we have reduced 16 of the 26 dosages to 8 (1500 IU, 2000 IU, 2500 IU, 3000 IU, 3500 IU, 4000 IU, 4500 IU and 5000 IU) by rounding them to the nearest 500 IU instead of 250 IU. These 8 dosages are commonly used and cover more than 90% of the patients. These admixtures have been assigned to Pharmacy Intravenous Admixture Service (PIVAS). Ten remaining dosages, between 1000–1250 IU and 5250–8500 IU, were used by less than 10% of the patients who were too heavy or too light, or having bleeding or clotting tendency. In the same exercise, these 10 specific dosages have been reduced to 5 ‘custom doses’ only. Now, the HD unit needs to make only these 5 ‘custom doses’. At this moment, they are needed by 12 specific patients only out of 200 patients. Their breakdown is: 1000 IU (2), 5500 IU (3), 6000 IU (3), 6500 IU (2) and 8500 IU (2). Viewed otherwise, now the HD unit only needs to prepare these specific dosages for an average of two patients per dialysis shift. A new protocol is issued for on-site dalteparin admixture. Under it, 0.5 mL (equal to 5000 IU) dalteparin is removed from the drug ampoule and mixed with 9.5 mL of 0.9% saline in a standard 10 mL syringe to make a 10 mL admixture (equal to 5000 IU). Tables on specific dalteparin doses and equivalent volumes of dalteparin–saline admixtures are displayed to guide the nurses. Under the tables, a specific dose of 1000 IU (equal to 2 mL) could be obtained by discarding 8 mL from a 10 mL admixture. All nurses have been retrained in various areas, including knowledge of anticoagulant use, technical skill in dalteparin admixture and other preparation process, double check, potential adverse effect and management, medication safety, incident reporting, etc. They have been taught and tested not only in their medical knowledge, but also taught, observed and assessed in their practical work to ensure over all competence. In order to minimise any distraction during medication preparation, a ‘do not disturb’ sign is displayed outside the clinic room.
Now the dalteparin orders are sent to PIVAS for the preparation of prefilled syringes ahead of each HD shift. Its work flow is shown in figure 2. Its staff will verify the orders and prepare the prefilled syringes accordingly. The patient’s unique hospital number is added to the drug labels to secure accuracy. Each day, the prefilled syringes will be delivered to the HD unit where they will be counted by two nurses. At the start of the HD, two qualified nurses will carry out double check and match the labelled syringes with the prescription orders and the correct patients at the bedside.
PIVAS workflow. HD, haemodialysis; LMWH, low-molecular-weight heparin.
Since we introduced our various new measures in the dalteparin admixture process, in the ensuing 4 weeks, we had watched very closely if there was any clot in the ECC or bleeding episodes in the HD unit. We found none. For 29 months in a row, no further incident arising from the preparation of dalteparin admixture has been reported.
Discussion
The use of LMWH such as dalteparin in HD is a common practice.2 3 Medication errors involving heparin are commonly reported.8 Its safe use hinges on many factors. Incident reporting is seen crucial and pivotal to initiate any safety improvement. However, reports on medication error has not been always carried out due to barriers on individual, organisational and cultural level.9 In China, incidents are not always reported and openly discussed for many reasons. One of them has been the traditional punitive approach to medication errors.10 Our hospital has been commissioned to bring about international practices and safety culture as part of healthcare reform in China.7 Among them have been incident reporting and the non-punitive approach to error reporting. They have enabled our staff to report incidents without fear of punishment and to learn from mistakes. Gradually, this concept of patient safety culture is taking roots and being acknowledged in China.11 This should help reduce medication errors and enhance patient safety.
Dosage miscalculations and numeracy errors are common in any hospital environment.12 In reducing our specific dalteparin dosages from 26 to 13 and their use discretion, we believe that we have succeeded in reducing our dalteparin medication errors to a very large extent. Our approach and experience could find support from the study of Donihi et al.13 The study found a significant reduction of medication errors and adverse effect after the standardised insulin sliding scales was reduced from over 20 to 3. It might be noted that independent double check is being used ‘judiciously’ in our HD unit because of time and manpower constraint. In any equation, patient safety is paramount. The Institute for Safe Medication Practices14 15 thought to certain extent that fewer double checks placed at the most vulnerable points will be preferable to an overabundance of double checks independent of each other. Our new dalteparin process seems to share its views to certain extent.
We have tried to avoid the recurrence of similar errors by reducing dosages’ number from 26 to 13 and their use discretion; asking PIVAS to prepare eight dosages commonly used, leaving only five custom doses to the care of HD unit; developing new protocol, tables, guide and workflow; retraining and reassessing our staff; and taking other measures. Yet, we must keep in mind that the dalteparin admixture process is a complex one involving different units and many hands, even a minor slip may lead to errors again. No vigilance is too much.
In shifting the dalteparin admixture and related work to PIVAS, we estimate that there is a saving of about 1 hour of nurses’ time per dialysis shift. This is the time which the nurses need to prepare for them on their own previously. While saving time for our nurses, it incurs a US65 cents per treatment for which the patient with HD needs to make an average of 15% co-payment, equivalent to US10 cents. It might be added despite the prefilled syringes prepared by PIVAS could contribute to patient safety considerably; presently, the overall percentage of Chinese hospitals with PIVAS remains very low at about 3.3%.16 When PIVAS scheme started, five patients raised queries about the extra charges. After dialogue with and explanation from doctors and nurses, all patients fully understood and accepted it. In compiling the 6 monthly general patient with HD satisfaction survey, patients were invited to complete a set of structured questionnaires and make comments. No more complaint was noted.
With this series of actions in place, no further incident in relation to preparation of dalteparin–saline admixture has been reported in the ensuing 29 months. Admittedly, there is limitation to our present study in that we cannot be sure if some numeracy mishaps or other errors involving dalteparin admixture and application might have gone unidentified during this period of time.12 Nevertheless, we believe that incidents of great magnitude affecting multiple patients should be avoidable.
Conclusions
Our report analysed how and why a LMWH incident occurred in our HD unit and what QI actions had been taken to address the issues. Our new and main approaches have been the reduction of the total number of dalteparin dosages and the division of labour and responsibility between PIVAS and the HD unit among others. Different dialysis units have different clinical experience and may recommend different anticoagulant and dose for their patients with HD. We believe our strategy could be converted into fewer anticoagulation errors and might be proven useful to other dialysis units.
Ethics statements
Patient consent for publication
Acknowledgments
Ms. Helen Chan and Ms. Lan-ping Shi
Footnotes
Contributors Q-zT, Y-fM, H-hJ, R-jX, YL, LL, L-wC and PP have all contributed to the conception or design of the work and its application. Q-zT, Y-fM and PP were involved in drafting the work and revising it critically for important intellectual content. All authors have read and approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.