Article Text
Abstract
Objectives Serious adverse effects, including arrhythmia and cardiac arrest, result from rapid intravenous high concentration of potassium chloride (KCl). We aimed to eliminate prescription of undiluted KCl and encourage dilution of KCl to 400 mEq/L and 40 mEq/L in the intensive care units (ICUs) and general and outpatient departments, respectively.
Methods Before the first intervention, we collected data regarding high-concentration KCl and interviewed representatives of physicians prescribing high-concentration KCl. Based on the guidelines in other countries on safely used concentrations of KCl (400 mEq/L), we negotiated with physicians to dilute KCl below 400 mEq/L. In the first intervention, we made rules based on surveys above. In the second intervention, we revised the rules based on opinions from physicians and pharmacists and investigated the change in the number of prescriptions of KCl concentration in each department. Continuing efforts with the safety manager ensured compliance of the rules by physicians and nurses in all departments.
Results After the first and second interventions, prescriptions for undiluted KCl in ICUs and general wards were eliminated (median=0). Prescriptions for <400 mEq/L KCl increased to 110 (median) after the first intervention and to 137 (median) after the second. In the general ward, 7 months after the first intervention, prescriptions for <400 mEq/L KCl had not increased. Compliance with our rules was high, and more than 72% of physicians and nurses were aware of the rules.
Conclusions The rules for administration of high-dose KCl successfully eliminated prescription of undiluted KCl, which was maintained using two plan-do-study-act cycles. Our intervention process could be useful in countries where prediluted formulations are unavailable or where prescriptions are not matched and undiluted ampules are used.
- pharmacy
- prescriptions
- potassium chloride administration
- adverse effect
- safety management
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Serious adverse effects due to rapid intravenous potassium chloride (KCl) administered at high concentrations include arrhythmia and cardiac arrest and these occur worldwide. The inadvertent administration of higher doses of KCl due to miscalculation is a substantial concern. There is greater concern in countries in which prediluted formulations of KCl have not been mass-produced, and in countries where undiluted ampules are used when prediluted formulations matched to a prescription are unavailable.1 2
The Joint Commission for Accreditation of Healthcare Organizations (JCAHO) reported 10 fatal accidents in the USA between 1996 and 1997 due to the administration of high concentrations of KCl.3 In Canada, 23 incidents related to high-dose KCl were reported from 1993 to 1996.3 In Japan, eight such incidents were reported from 2009 to 2015.4
In Australia, Canada, the UK and the USA, undiluted KCl ampule products are treated as high-risk drugs.3 5 6 The National Patient Safety Agency, JCAHO, and the WHO have produced guidelines for handling high concentrations of KCl, as follows5–10:
KCl ampule products are prohibited from being stored in wards and outpatient departments.
KCl preparations are prohibited in wards and outpatient departments.
Prediluted formulations of 100–400 mEq/L can be adopted.
Optimal concentrations should be decided, limited and standardised.
Although these recommended measures are being implemented in various countries1 11–15; in some cases, restricting the storage of undiluted KCl ampules in wards and outpatient departments has been the only effort made to follow these measures.
Prediluted formulations of KCl are not available in all countries. In these situations, KCl ampule products and intravenous drip formulations are delivered to healthcare settings without prior mixing. This means that physicians and nurses must dilute and prepare KCl in healthcare settings. Worldwide, there is a lack of standardisation of the recommended safe concentrations for use in the hospital, which means that potentially dangerous doses continue to be administered in hospitals in some countries.
Unfortunately, some physicians, especially junior residents, at our hospital have prescribed high-dose or undiluted KCl for patients with no clinical indication for high-dose KCl. Our hospital pharmacists were hesitant to question physicians regarding prescriptions that high-concentration KCl because they are aware that some patients may need higher doses.
Based on our interactions with pharmacists in few Japanese hospitals regarding high-concentration KCl prescriptions, it was considered that an increasing number of physicians were prescribing and administering high-concentration and even undiluted KCl for patients who did not need such high concentrations.
Therefore, we aimed to establish a system of administering high-dose KCl to patients who need highly concentrated doses in a clinical setting. The following rules were decided on: physicians can dilute undiluted injectable KCl to a concentration of up to 400 mEq/L, which can then be safely administered. For patients who do not need high-concentration doses, physicians should dilute KCl to concentrations below 40 mEq/L, as stated in the attached Japanese document.
Methods
Setting up the task team
A task team was created that included physicians, pharmacists and nurses representing the quality and safety management, nursing and pharmacy departments. We conducted the surveys shown in the online supplementary table before the intervention.
Supplemental material
In Japan, no papers or guidelines have discussed the use of high concentrations of KCl in clinical practice. Even in nearby healthcare facilities, KCl concentrations are not standardised. For assistance with our interventions, we referred to the guidelines published in other countries. The number of undiluted prescriptions between April 2015 and October 2015 was 72 (median) for intensive care units (ICUs) and four (median) for the general wards and outpatient departments. This was taken as the baseline. Our intervention had three aims: (1) to set the prescription number of undiluted KCl to 0 in all situations and in all departments; (2) to ensure that, in ICUs, KCl is always diluted to 400 mEq/L (maximum concentration); and (3) to ensure that, in general wards and outpatient departments, following the guidelines of the attached Japanese document, KCl is always diluted to a concentration of 40 mEq/L (maximum concentration).
Based on our interviews with physicians in some clinical departments who prescribe high KCl concentrations, we determined that concentrations of up to 400 mEq/L could be used, but only for patients who need highly concentrated KCl because of specific clinical conditions.16–20 We presented our case to physicians in the clinical departments that were prescribing highly concentrated KCl, after we conducted interviews and surveys with hospital staff and physicians in overseas countries. Furthermore, we reviewed the healthcare guidelines of these other countries, which indicated that KCl should be diluted to below 400 mEq/L for safe administration. The responses we received from all the physicians indicated that doses up to 400 mEq/L can be safely administered, particularly in patients requiring highly concentrated KCl.
First intervention
Based on the results of the surveys, including interviews with physicians, survey of data regarding number of prescriptions of high concentrations of KCl, and survey from nearby healthcare facilities, we developed rules 1–5, which are listed in table 1.
Rules of the first and the second interventions
Modification of the KCl warning document provided to nursing staff
Prior to implementation of Rule no. 4 and allowing pharmacists to provide KCl notifications to the nurses, the task team reviewed the contents of the warning document provided to the nursing staff. We modified the document to state that if the prescribed dose of KCl exceeded 40 mEq/L, the nurse should confirm the KCl concentration with the physician in the general ward or outpatient department.
Before implementing the rules, we requested permission to work with all physicians’ and nurses’ safety managers in each clinical department, ward and outpatient department. As they were working with us on medical safety initiatives, the safety managers did not offer any objection to the rules. On confirmation of the safety managers, we began implementing the rules in October 2015.
After effectuating the new rules, we found that they were accepted by the hospital staff without difficulty; nevertheless, approximately 10 months after the rules had been in force, physicians and pharmacists suggested that it was necessary to modify part of the rules.
The physician who managed the templated prescriptions in the ICUs stated that it was necessary to administer KCl in high concentrations to patients with a medical history of cardiac insufficiency who were admitted in clinical areas other than the cardiovascular surgery, critical care and cardiovascular departments. This physician also suggested that if high-dose KCl could be administered to patients who required potassium and fluid restriction because of cardiac dysfunction in the ICUs, then concentrations of up to 400 mEq/L should be allowed for such patients in all clinical departments. The physician further noted that dialysis is performed not only in the dialysis room, but also in ICUs. Therefore, concentrations of up to 400 mEq/L should be allowed for patients undergoing dialysis in ICUs.
The pharmacists opined that when potassium was administered in an intravenous drip formulation, it would be challenging to comply with doses below 40 mEq/L. They suggested that concentrations over 40 mEq/L should be allowed in certain cases, even in the general ward and outpatient departments.
Presentation of rules and guidance to hospital staff
To initiate the intervention, we communicated with and educated all staff through the safety manager. Since 2015, we have been working continually with safety managers to check that compliance with the rules is being maintained, with regards to physicians in all departments and nurses in all wards and outpatient departments. We revisit all departments every year and interview the staff regarding their awareness and whether they follow the rules. The results of the above are relayed back to each department, and the safety manager is enlisted to educate staff continuously.
Second intervention
The rules were modified during the second intervention in August 2016 (table 1). We held a task team meeting to discuss whether the opinions and suggestions received from physicians and pharmacists were appropriate. Because physicians’ opinions were clinically based, we decided to recognise them and make changes to the previous rules.
We accepted the opinions from pharmacists with some restrictions. Finally, we decided that for any patient who was prescribed KCl in excess of 40 mEq/L in the general ward or outpatient department, the pharmacist must communicate with the physician about the dosing. If, together, the physicians and pharmacists judge administration of over 40 mEq/L KCL to be acceptable, the prescription should be filled.
After the first and second interventions, we assessed and monitored the effects of the rules by evaluating: (1) the change in the number of undiluted and 100–400 mEq/L KCl prescriptions written in ICUs and the general ward and outpatient departments, (2) the change in the number of prescriptions for 40–100 mEq/L and the number written for less than 40 mEq/L KCl in the general ward and outpatient departments and (3) the change in the number of prescriptions that were altered due to inquiries made by pharmacists in all wards and outpatient departments.
Approval by an independent local, regional, or national review board
Approvals were not obtained from independent local, regional, or national review boards because we developed our own rationale for the safe administration of injectable high-concentration KCl; thus, these approvals were not deemed necessary.
Patient and public involvement statement
Neither patients nor the public were involved in the design of this study because we developed our own rationale for the safe administration of injectable high-concentration KCl; thus, it was not necessary for these groups to be involved in the design of this study.
Results
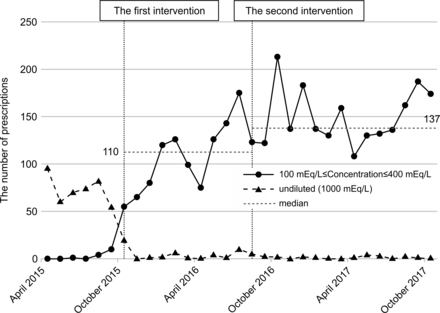
In the ICUs, the number of prescriptions for undiluted KCl was 72 (median) before the first intervention (figure 1). This number decreased to 0 (median) after the first intervention and remained at 0 (median) after the second intervention. Conversely, the number of prescriptions for 100–400 mEq/L was 0 (median) before the first intervention and increased to 110 (median) between the first and second interventions and to 137 (median) after the second intervention.
Changes in the number of prescriptions for undiluted potassium chloride (KCl) in intensive care units (ICUs) and the number of prescriptions for 100–400 mEq/L KCl. In the ICUs, the number of prescriptions for undiluted KCl was 72 (median) before the first intervention. This number decreased to 0 (median) after the first intervention and remained at 0 (median) after the second intervention. Conversely, the number of prescriptions for 100–400 mEq/L KCl was 0 (median) before the first intervention, increasing to 110 (median) after the first intervention and 137 (median) after the second intervention. The baseline was evaluated between April 2015 and October 2015 and is shown in the figure.
In the second intervention, the previous restrictions of prescriptions for 400 mEq/L KCl to specific clinical departments only was modified to allow prescriptions for up to 400 mEq/L in all clinical departments. After this intervention, the number of prescriptions for 100–400 mEq/L KCl increased; however, the number of undiluted prescriptions did not increase.
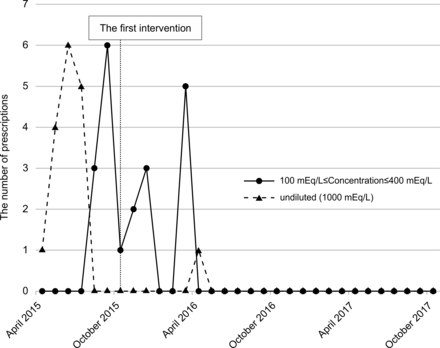
In the general ward and outpatient departments, the number of undiluted prescriptions was 0 at 7 months after implementation of the first intervention (figure 2). Several prescriptions were written for 100–400 mEq/L KCl, which was allowed only in the ICUs, before the first intervention. Nevertheless, this number decreased to 0 by 6 months after implementation of the first intervention. After the second intervention, this number remained at 0.
Changes in the number of prescriptions for undiluted and 100–400 mEq/L potassium chloride (KCl) in the general ward and outpatient departments. In the general ward and outpatient departments, the number of prescriptions for undiluted KCl decreased to 0 within 7 months of implementation of the first intervention. Prescriptions for doses up to 400 mEq/L, which were permitted in a limited number of situations such as the intensive care unit, did not increase. The baseline was evaluated between April 2015 and October 2015 and is shown in the figure.
The number of prescriptions for doses less than 40 mEq/L was 152 (median) before the first intervention, increasing to 200 (median) between the first and second interventions, and increasing further after the second intervention to 217 (median).
The number of prescriptions for 40–100 mEq/L was 34 (median) before the first intervention, but decreased to 12 (median) between the first and second interventions, then increased after the second intervention to 42 (median) (figure 3A).
(A) Changes in the number of prescriptions for 40–100 mEq/L and less than 40 mEq/L potassium chloride (KCl) in the general ward and outpatient departments. The number of prescriptions written for doses less than 40 mEq/L increased in the general ward and outpatient departments after the first intervention and remained higher than baseline after the second intervention. The baseline was evaluated between April 2015 and October 2015 and is shown in the figure. (B) Details of the proportions of prescriptions for 40–100 mEq/L KCl in the general ward and outpatient departments. The proportion of prescriptions for 40–60 mEq/L KCl in the general ward was 81% before the first intervention, increasing to 94% after the first intervention, and then decreasing to 66% after the second intervention. The reason for the increase after the first intervention was that dosing in excess of 40 mEq/L was allowed, according to modified Rule number 3 (additional exclusion criteria). When physicians and pharmacists agreed that a dose exceeding 40 mEq/L of KCl was necessary, it was allowed.
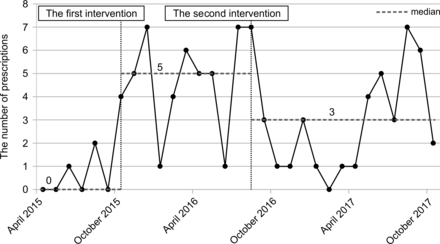
Before the first intervention, the proportion of prescriptions for doses that exceeded 40 mEq/L KCl were: 40–60 mEq/L: 81% and 60–100 mEq/L: 19%. Between the first and second interventions, the proportions were: 40–60 mEq/L: 94% and 60–100 mEq/L: 6%. After the second intervention, the proportions were: 40–60 mEq/L: 66% and 60–100 mEq/L: 34% (figure 3B). The number of prescriptions that were changed based on pharmacists’ inquiries was 0 (median) before the first intervention, five (median) between the first and second interventions, and three (median) after the second intervention (figure 4).
Number of prescriptions changed based on pharmacists’ inquiries. The pharmacists’ method of inquiring about potassium chloride (KCl) prescriptions was clarified by the intervention, and their readiness to inquire about KCl prescriptions that deviated from the rules improved after the first intervention. We considered that the number of prescriptions changed because the pharmacists’ inquiries increased again after April 2017, as there was a large physician turnover at that time in our hospital, and these physicians were likely not aware of the rules. The baseline was evaluated between April 2015 and October 2015 and is shown in the figure.
The results of our assessment on the awareness of the rules regarding recommended concentrations and cautions for administration of KCl following the first intervention (starting from 2015) were as follows: on average, 72% of physicians knew the rules in 2015, 86% did in 2016, 84% did in 2017 and 84% did in 2018. On average 97% of nurses knew the rules in 2015, 89% knew in 2016, 89% knew in 2017 and 93% knew in 2018.
Discussion
In the ICUs, the first intervention led to a change from using undiluted prescriptions to prescribing doses of KCl that were diluted to a maximum concentration of 400 mEq/L (figure 1). In the second intervention, we modified the rules so that doses up to 400 mEq/L KCl were permitted in all departments; the number of undiluted prescriptions did not increase after the second intervention. The reason for the increase in use of 60–100 mEq/L KCl in the general ward and outpatient departments after the second intervention was the ambiguity of the modified Rule number 3 (additional exclusion criteria), which stated “When physicians and pharmacists agree that a dose exceeding 40 mEq/L KCl is necessary, it is allowed”. When 1000 mL of replacement fluid is administered, as is often carried out in general wards and outpatient departments, KCl concentrations below 60 mEq/L are recommended.19 Because we had not specified the upper limit of the KCl concentration in the modified rule, the pharmacists began to deliver concentrations exceeding 60 mEq/L KCl.
In the general ward and outpatient departments, the number of prescriptions for undiluted KCl decreased to 0 within 7 months of implementation the first intervention (figure 2). The number of prescriptions for doses up to 400 mEq/L, which were permitted in a limited number of situations such as in the ICUs, did not increase. From these data, it was considered that both the first and second interventions were effective.
The number of prescriptions written for doses less than 40 mEq/L increased in the general ward and outpatient departments after the first intervention, and was higher after the second intervention than it was before the first (figure 3A).
Despite both interventions, it was not possible to reduce the number of prescriptions for doses of over 40 mEq/L KCl to 0. One reason for this could be the modification to Rule no. 3 in the second intervention, which allowed prescriptions for doses exceeding 40 mEq/L KCl if it was agreed on by the physician and the pharmacist. However, all prescriptions for doses over 40 mEq/L KCl were below 100 mEq/L after both interventions. Doses of KCl less than 100 mEq/L can be safely administered, as this is the concentration that can be given via peripheral intravenous, and KCl is marketed as a prediluted product in some countries.
The proportion of prescriptions for 40–60 mEq/L KCl in the general ward was 81% of total prescriptions before the first intervention, increasing to 94% between the first and second interventions, and then decreasing to 66% after the second intervention (figure 3B). We believe that the reason for the increase after the first intervention was the permission of dosing in excess of 40 mEq/L according to physician and pharmacist consensus.
Seven months after the first intervention, prescriptions above 100 mEq/L KCl accounted for 0% of the total prescriptions in the general ward and outpatient departments (figure 2). This suggests no serious concern regarding the safe use of KCl.
Pharmacist inquiries increased, which confirmed the appropriateness of the rule that requires pharmacists to inquire about all prescriptions that deviate from the rules (figure 4). The pharmacists’ method of inquiring about KCl prescriptions was clarified by the intervention, and their ability to inquire about KCl prescriptions that deviate from the rules improved after the intervention.
After the first intervention; when the pharmacists began to inquire about all the prescriptions deviating from the rule, the number of prescriptions changed because the number of inquiries increased to five (median). Based on the premise of the second intervention, it was hypothesised that pharmacists’ inquiries about physicians’ prescriptions might result in fewer changes to prescriptions. Our suppositions were somewhat realised, as the number of prescription changes caused by pharmacists' inquiries was only three (median). We presume that the number of prescriptions changed due to pharmacists' inquiries increased again after April 2017 because there was a large physician turnover at that time in our hospital, and these physicians were likely not aware of the rules.
Nevertheless, there appears to have been no difficulty in modifying the rules by implementing the second intervention, because the number of prescriptions for high-dose KCl did not increase (figures 1 and 2).
Overall, hospital staff compliance with the rules to eliminate undiluted KCl prescriptions was almost 100%. This resulted from the combined efforts of the clinical department of quality and safety management, and a task team comprising members of the nursing and pharmacy departments.
Our objective in this study was to implement interventions to minimise severe complications due to high-concentration KCl prescriptions. Even in the absence of adopting prediluted formulations, we were able to reduce the number of undiluted prescriptions in our hospital to 0 (median) by implementing these two interventions. As part of our interventions, we were able to accomplish several important tasks: 1) we effectively changed templated KCl prescriptions in the ICUs from undiluted KCl to 400 mEq/L KCl; 2) in the general ward and outpatient departments, our first intervention reduced the number of prescriptions for undiluted KCl to 0; 3) the number of prescriptions for doses up to 400 mEq/L, which were permitted in a limited number of situations such as in the ICU setting, did not increase; 4) the number of prescriptions written for doses under 40 mEq/L increased in the general ward and outpatient departments; and 5) we standardised pharmacists' inquiries relating to prescriptions for high-dose KCl, and established a system so that nurses would receive a warning document describing the details of the KCl concentration at the time of administration.
For most rules, the management of KCl concentration by hospital staff was changed based on our intervention, and we believe that our intervention could be useful. The implementation of our rules for administration of high-dose KCl at our hospital successfully decreased the prescription of undiluted KCl, which was maintained through two plan-do-study-act cycles.
We believe our intervention would be useful in other countries that also do not have prediluted formulations and where prediluted formulation matched to prescription is unavailable, and undiluted ampules are used instead.
Limitations
The reason that the number of prescriptions written for concentrations above 40 mEq/L KCl did not decrease in the general ward and outpatient departments after the second intervention was attributable to the low number of hospital beds in our ICU. We also suppose that patients needing potassium and fluid restriction due to cardiac dysfunction could not be transferred to the ICU.
Future directions
In the ICUs, most prescriptions were diluted to maximum concentrations of 400 mEq/L, but some prescriptions remained undiluted. Therefore, we need to reinforce our rules. It is possible that, because more than 3 years have passed since the rules were implemented, documents describing the rules are not being reviewed by hospital staff. It is necessary to periodically reinforce these rules among physicians, nurses and pharmacists via hospital news, communications and training sessions. To encourage adherence to guidelines and regulations, we plan to routinely monitor hospital staff compliance with the rules, obtain staff opinions and revise the rules as necessary.
Henceforth, we will check prescription data two times per year to assess whether the intervention outcome described above has been maintained and that the status has not changed since October 2017. In addition, we plan to check pharmacist inquiry records every 6 months to check for deviation from the rules.
We plan to modify the rules by setting the upper limit as less than 60 mEq/L KCl for the general ward and outpatient departments.19 We plan to implement this in our hospital staff, and will routinely investigate compliance with this rule. The safety manager has been instructed to report any problems that the staff encounter with regards to diluting KCl, and suggestions, if any, for improvement of the rules.
Based on the results of this study, we will consider increasing the number of beds in the ICU.
We propose that the guidelines in the attached document should be modified to allow up to 400 mEq/L of KCl in certain clinical situations. In addition, until prediluted KCl formulations are made available in most countries, we propose that KCl ampules and intravenous drip formulations should only be mixed by pharmacists in the pharmacy departments prior to delivery to the various healthcare settings. Limiting the preparation of KCl to pharmacists would provide an added safety measure in countries where prediluted KCL formulations are not yet available.
Acknowledgments
I am grateful to Mr Kazunori Nakamura and Ms Kumiko Fujinaga for
their helpful discussions.
Footnotes
Contributors KN designed the study and wrote the initial draft of the manuscript. EN-Y, as a PI of this study, contributed to the analysis and interpretation of the data and assisted in the preparation of the manuscript. YS contributed to conducting the project, data collection and interpretation related to the study. ST and TN critically reviewed the manuscript. TN also supervised the entire study process.
Funding The study was supported by Osaka City University Support Office for Female Researchers.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement All data relevant to the study are included in the article or uploaded as supplementary information.