Article Text
Abstract
Background Prostate cancer (PC) is the second most common cause of cancer deaths among males worldwide. Prostate-specific antigen (PSA) is a predictive indicator of prostate pathology. Men with elevated PSA levels are at increased risk of developing PC. There is currently no UK national PC screening programme, therefore patients often present to general practices (GPs) at later stages of pathology, worsening patient prognosis and outcomes.
Local problem The location of the GP surgery had a large patient population at increased risk of PC, namely Afro-Caribbean/Asian males.
Methods We conducted baseline measurements to identify male patients over the age of 65 and/or male patients who were at high risk of developing PC. These included previous referred patients or patients with a PSA over 10.0. We then implemented three plan-do-study-act (PDSA) cycles and measured their effect after 2 weeks of starting the respective intervention.
Interventions PDSA1: Generating a list of target patients who have not had repeat/follow-up/referral and directly contacting by telephone to invite them for a blood test.
PDSA2: Creating patient-specific electronic pop-up reminders on the electronic-patient-record system for PSA follow-up/referral/repeat test.
Planned PDSA3: Patient education of prostate health and general self-checking, as well as benefits/risks of undergoing PSA screening in the form of patient focus groups and informative leaflets.
Results We identified 220 male patients over 65 registered at a large South London GP surgery. 77.7% of eligible patients had a PSA measurement since 1 April 2019. Our results showed an overall increase in screening of 13.5% from baseline.
Conclusions Our project identified patients that may potentially have undiagnosed prostate pathology. However, a key factor for not reaching our goal was blood test refusal. This was further exacerbated by the COVID-19 pandemic, impacting the capacity to disseminate appropriate information to the local population on the importance of PSA screening.
- general practice
- continuous quality improvement
- patient education
- health equity
- PDSA
Data availability statement
All data relevant to the study are included in the article.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
This paper describes the outcomes of a quality improvement project (QIP), conducted at a South London general practice (GP) by fourth year medical students supervised by practice staff. This project was guided by SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines in quality improvement.1
Problem
Prostate cancer (PC) is the second most common malignancy in men worldwide, causing over 355 000 deaths in 2018.2 The lack of UK national cancer screening programme leads to patients presenting at later stages of pathology which may worsen patient prognosis.3 Prostate-specific antigen (PSA) testing, although present with issues regarding specificity, can be a useful screening test to assess prostate pathology.
In terms of current condition mapping, the GP lacked a standardised protocol to measure PSA levels. The practice referred high-risk patients based on clinical decision making but believed this was inadequate due to the raised risk of PC in their patient demographic. This project aimed to introduce interventions to detect at-risk patients at earlier stages of pathology or increase missed patients’ referrals to urology.
Our root cause analysis identified key factors contributing to the local problem. Patient and social factors include socioeconomic demographics; with two major risk factors for PC being over the age of 65 years and Afro-Caribbean ethnicity in the catchment population.4–6 It is highly likely that a project increasing the uptake of PC screening would be beneficial to the local population.7 Key patient factors included lack of patient understanding on PC screening, resulting in high rates of investigation refusal. Task factors include the lack of PSA-specific protocol and time constraints on clinicians.
Between 1999 and 2002, the annual PSA-testing rate was approximately 6% in the UK.8 9 Compared with national cancer screening programmes (breast, bowel) that have uptakes of over 60%, it is evident that PSA testing is significantly lower.10
Key benefits of PC screening include identifying prostate pathology at earlier stages, therefore improving PC-specific disease outcomes. There are also a number of concerns regarding overdiagnoses, overtreatment and extensive investigative tests such as biopsies.11 National health bodies have chosen not to use PSA testing as part of the national cancer screening programmes. Further research may be required to identify the appropriateness of only including PSA testing at high-risk GP patient populations.
Aims
Our aim was to ensure >95% of male patients over the age of 65 or those at high risk of developing PC, currently registered at a South London GP have had a PSA screening test since 1 April 2019; or have been referred if abnormal by March 2021. We aim to achieve this using long-term sustainable methodology.
Methods
Design
The team included administrative staff who assisted with storage of confidential patient documents and electronic-patient-record (EPR) training, two general practitioners who supervised the creation of eligibility criteria and guided the project. Neil Limaye, Daniele Zorzato and Aaruran Nadarajasundaram planned and carried out the project, measured the effectiveness of each cycle, interpreted and wrote up the project.
Our root cause analysis identified key factors for the reduced PSA screening at this practice. We designed a three-intervention protocol to address this directly. Intervention 1 aimed at optimising organisational factors by creating a user-friendly database of high-risk patients. This would minimise additional time constraints on clinicians by streamlining patients who may benefit from additional monitoring. Intervention 2 targeted organisational factors using EPR reminders to reduce environmental costs and improve long-term patient monitoring. Intervention 3 targeted patient factors directly by addressing the lack of understanding around PC screening.
Baseline measurement
First, we created eligibility criteria for patients in this study: (1) male patients over the age of 65 and/or (2) being at high risk of developing PC and (3) patients who have not had a test since 1 April 2019. This timeframe was chosen based on Quality and Outcomes Framework (QOFs) used in primary care.12 High-risk patients were those with previous PSA results >10.0 ng/mL and/or a previous referral to urology for suspected PC. This was based on guidelines that suggest asymptomatic patients with a PSA >10.0 ng/mL require 2-week wait referrals.13
We completed a baseline measurement of eligible patients over a 2-week period using the Vision EPR system at the GP. After satisfying the eligibility criteria, we identified 220 at-risk patients. We further reduced the identified baseline patients from 220 to 171 as only 49 patients were untested in the appropriate time frame.
Interventions
This project involved three plan-do-study-act (PDSA) cycles to assess impacts on PSA screening uptake.14 The PDSA timescales were designed based on the medical school academic year. We implemented three cycles lasting 2 weeks each, measuring impacts at cycle completion.
PDSA1 (21 October 2020–25 November 2020)
Plan: PDSA1 identified eligible patients and created a paper patient list against which future interventions can be compared against.
Do: The intervention involved contacting patients by telephone for a short consultation to invite them for a PSA test. We predicted this will increase the uptake of at-risk patients.
Study: There was an increase in the uptake of patients for PSA screening from 171 to 185 (+8.2%) The predicted % increase for this PDSA cycle was 8.0%, indicating a greater than predicted uptake.
Act: Although effective, this intervention was labour intensive and environmentally unsustainable. A future modification includes automating the patient list electronically to improve resource allocation, practice efficiency and reducing environmental impact. This led us to create PDSA2, an electronic intervention.
PDSA2 (25 November 2020–9 December 2020)
Plan: PDSA2 involved the introduction of a pop-up reminder on EPR for each eligible patient.
Do: Based on eligibility criteria, we identified patients benefitting from a repeat test. We entered this information into the EPR pop-up notification system to remind respective clinicians. We predicted this PDSA cycle to cause a smaller uptake as it is a more indirect intervention.
Study: There was an increase in screening uptake of patients from 185 to 194 (+5.3%). The predicted % increase for PDSA2 was 7.0%, indicating a lower than predicted uptake.
Act: Clinicians noted the EPR reminders allowed them to facilitate discussions with patients regarding PSA testing, finding low levels of patient PC understanding. This allowed us to develop plans for patient-based focus-groups to encourage patient education.
PDSA3 (20 January 2021–10 February 2021)
Plan: PDSA3 aimed to increase patient education of prostate health as well as benefits/risks of undergoing PSA screening. This involves ascertaining current patients’ understanding, encouraging discussions/questions and rectifying misconceptions regarding PSA testing.
Do: We planned to implement this using informative leaflets and patient focus groups. We predicted an uptake value of 7.2% because it was directly involving patients and providing patient education. This intervention may lead to sustained long-term benefits due to its impact on future patient adherence to interventions, despite a lower short-term impact.
Study: PDSA3 was one that was planned but not implemented due to the COVID-19 pandemic and lockdown restrictions.
Act: The embedding of this intervention would be to organise a yearly focus group meeting on a specific pre-planned date for patients of the local community.
Strategy
The impact of interventions was measured by nominal increase in uptake of PSA screening. This allowed us to compare the effectiveness of each PDSA cycle and specific intervention.
Our run chart (figure 1) was regularly updated with a timeline for each intervention to establish observed outcomes and the effect on increasing PSA uptake. The run chart served a secondary purpose to monitor study progress.
Shows a run chart of the impact our interventions had on the cumulative number of patients that have undergone a serum PSA screening. The goal of 209 patients is based on 95% of males over the age of 65 registered to the surgery. At baseline, 171 men had already undergone a serum PSA level measurement in the appropriate time frame. PDSA, Plan-Do-Study-Act; PSA, prostate-specific antigen.
Methods of analysis
Quantitative methods in this project involved the collection of numerical data at baseline and calculating percentage increase after each intervention.
Studies have demonstrated the benefits of utilising both quantitative and qualitative methodology to understand complex interventions and health systems.15 In our study, the proposed qualitative methods involved the organisation of patient focus groups. These qualitative methods would have assisted in understanding diverse patient perspectives and informed patient decision making for future change.16
Patient and public involvement
Patients were not directly involved in the design, or conduct, or reporting, or dissemination plans of this project.
Results
The implementation of PDSA1 led to an increase in uptake of patients from the baseline measurement of 171 patients on 21 October 2020 to 185 patients by the 25 November 2020 (+8.2%) (table 1, figure 1). This was the most effective intervention applied with the largest increase in uptake observed in week 7. PDSA2 demonstrated an increase from 185 to 194 patients. This resulted in an increase in nine patients screened (+5.3%), a lower value than PDSA1 (table 1, figure 1). Overall, PDSA1 and PDSA2 combined were responsible for a cumulative increase in 23 patients (+13.5%).
Summary of PDSA cycle measurements and effectiveness of interventions
The proposed PDSA3 could not be implemented due to the national lockdown restrictions applied from 21 December 2020 which extended to 8 March 2021. Our expected prediction and outcome from PDSA3 was to yield the highest increase in long-term patient uptake for PSA testing and to deliver the most benefit to community through patient education at a low opportunity cost.
Over the course of the project, there was an increase in 23 patients (+13.5%) with a total patient population screened of 194 patients (table 1, figure 1). This represented 88.2% of at-risk patients registered at the practice who underwent PSA testing, a value under the objective target of 95% due to contextual factors. Overall, the number of untested at-risk patients decreased from 49 to 26 after the completion of our PDSA cycles.
Discussion
Interpretation
Our project targeted 95% of patients screened for PSA testing but the results demonstrate the actual total of patients screened was 88.2% of males over 65 registered to the practice.
PDSA1 was more effective than PDSA2 (% increase=8.2%> % increase=5.3%-table 1) as it was more direct compared with passively relying on clinicians opening EPR to see the prompt. Telephone consultations have shown to be more cost-effective, efficient and accessible for patients making this intervention beneficial to increase uptake.17 Telephone counselling methods for cancer screening has been demonstrated to increase patient uptake through informed patient knowledge.18 19 Empowering patients with knowledge is often the most efficient and sustainable method to ensure adherence.
PDSA2 used electronic prompts at the point of care which have shown to improve care in a sustainable, scalable manner. PDSA2 may have greater long-term potential as changes are embedded but the 4-month timescale of the project did not allow us to observe this. Literature has shown clinicians responded to >60% of prompts and documented discussions for another 26.8%; considering EPR-prompts to be useful clinical reminders for clinicians.20 Guiriguet et al investigated the impact of EPR notifications in GP, concluding that these had a statistically significant impact on increasing uptake for cancer screening.21
The proposed PDSA3 intervention would have increased patient education in the local community with studies demonstrating educational interventions increase participation in cancer screening programmes; especially in areas with low literacy.22 Health education interventions achieved this by increasing patient knowledge, perceptions and self-efficacy.23 This intervention would have had a significant impact on the local patient population as the practice was located in a deprived area of London. Patients with lower annual incomes are less likely to attend cancer screening programmes which may be a factor affecting the efficacy of interventions applied in this area.24 PDSA3 targeted this as a strategy to increase uptake.
Studies demonstrated there is a reduced uptake of PSA testing in Asian and black populations.8 25 This coupled with Afro-Caribbean men being at greater risk of PC allowed our project to target the highest risk patients of society.6
We predicted the largest uptake of patients screened in PDSA1, with smaller but more long-term increases in uptake with PDSA2 and PDSA3. There were a variety of reasons why we did not reach the 95% target we had set. First, there are well documented issues in primary care surrounding the adherence of patients attending PSA testing and other investigations.26 This has been compounded by the COVID-19 pandemic, which saw an overall reduction in patients attending cancer screening programmes which may be explained by high patient anxiety and reluctance to attend healthcare services.27
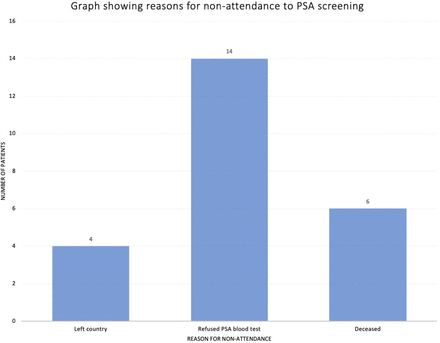
The pandemic limited the capacity of hospital and GP-based services with only urgent cases prioritised. Patient refusal rates were a key factor in this study with 14 patients declining the PSA test (figure 2). PDSA3 could have improved this refusal rate by increasing patient education on PSA screening.
Visual representation of the reason for non-attendance to PSA level measurements. The most common reason, with 14 individuals, was refusal of serum PSA levels and general blood tests, followed by death and not being in the country with 6 and 4 individuals, respectively. PSA, prostate-specific antigen.
Lessons and limitations
This project included multiple challenges, many of which were focused on the nature of a student-led QIP. Nevertheless, each limitation proved valuable learning experiences and ensured long-term project sustainability.
The time frame of this study was a key limitation as our methodology only allowed for the collection of 4–6 months of data. However, as the interventions applied were embedded, this promoted long-term patient benefits, meaning some interventions may have been further successful in future cycles. To further the project, the practice will be able to use the framework we developed for continued cycles over a prolonged time frame. This would allow for better understanding of the benefits of each intervention and increase the quantity of data collected.
Another limitation was that data collection was only accessible at the practice. In contrast, this was beneficial as it did not introduce any bias due to our group having no direct involvement with patients.
Familiarising the group with the EPR system took valuable time at the start of the project to create baseline measurements. However, this is a one-off limitation as once the familiarisation occurs, subsequent implementations are relatively straightforward.
The local population was also highly populated with Asian and Black minority populations which may not show a representative marker for the national population. Despite the small sample size and population demographics, our QIP can provide valuable information on PC screening, specifically in high-risk population groups.
Financial, environmental and social costs were considered in the PDSA cycles and interventions applied. There was an initial cost of resource allocation for the practice selecting a supervisor. PDSA1 involved a physical cost of time and labour in identifying, generating the list, contacting and counselling individual patients to invite them for a PSA test. A key limitation to the sustainability of this intervention is the active maintenance of the list and the addition of new joining patients entering the at-risk group. In contrast, this intervention was beneficial in understanding the initial scale of the project, giving an overview of the high-risk patients who needed focused interventions. This facilitated implementation of PDSA2 and PDSA3 by providing us with valuable feedback.
PDSA2 was labour intensive and time-consuming as records needed to be manually accessed and edited individually. Additionally, each file needed to be specifically accessed to view the prompts, potentially compromising the effectiveness of the prompt. Nevertheless, the cost trade-offs were minimised as EPR software was already present and data could be easily extracted and edited. These prompts would encourage discussions regarding PSA testing for high-risk patients who may have otherwise be missed.
The proposed PDSA3 involved patient focus groups which may have required patients to take time to come to the GP which also creates a cost to the GP in terms of finances and time allocation.
Sustainability
Sustainability is a fundamental aspect to this study and of any public health intervention. This project ensured that the interventions we applied had potential to be scaled up while maintaining low overall costs. PDSA1 created a lean-service delivery to streamline care to high-risk patients into a directly identifiable list which can be easily maintained and used as a model at other practices.28 Using lean-management techniques can improve patient care and satisfaction, reduce costs and time delays in referrals.29
The PDSA2 online EPR notification system was a clear and concise reminder for PSA testing and is a low-carbon alternative. This was a useful, scalable, and embedded intervention for continuity of care, as repeat PSA testing notifications could be placed for each patient to monitor progress. A key advantage to this intervention is its long-term benefit beyond the end date of our project, making it sustainable and valuable to patients in the long-term. Although many clinical practices use different EPR systems, we recognise the potential for this intervention to be scaled locally and nationally.
Finally, the proposed PDSA3 would have increased patient education making it the most directly beneficial for the local community; involving prevention, patient empowerment and self-care.28 30 This is a crucial aspect of helping patients understand the benefits of PSA testing, increasing patient health self-awareness and health promotion. This intervention ensures that the other two interventions are successful by increasing patient awareness and compliance to the screening programme. This maintains patient autonomy in patient-centred care but gives them the understanding to make independent decisions. In the future, this intervention can be embedded sustainably by creating a protocol that allows for the flexibility of the intervention to be delivered in person or remotely.
In terms of sustainable value in healthcare, our project redefined value by focusing high-value processes on patients at greater risk, balancing good quality patient care and resource allocation.28 We identified frustrations encountered with sustainability, including the improvement evaporation effect (IEE), whereafter significant improvement is implemented, there is a return to the original level. The IEE encourages the adequate embedding of interventions for long-term sustainable patient care.31 To prevent the IEE, we involved all relevant stakeholders, ensuring they understood each intervention carefully. This was achieved by effectively communicating with the GP staff throughout the project, especially during development of protocols. Furthermore, utilising pre-existing EPR technology avoided retraining and developing new skills that may hinder long-term staff compliance.
Conclusion
Our PDSA cycles improved patient care and clinical outcomes by increasing patients screened. This could help identify patients at higher risk of prostate pathology thereby increasing their long-term quality of life. We also considered a cost-benefit analysis to measure financial, environmental and social impacts.32
This QIP highlighted the need to invest more resources into PC screening. As this project was based at a local GP level, it is difficult to extrapolate this data to a national setting. Further studies could research the cost and clinical effectiveness of PSA screening and the potential of only targeting high-risk patients. Coupled with our suggested sustainable PDSA interventions, this may prove a successful combination to reduce PC deaths in the future.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Ethics approval
Formal ethical approval was considered and deemed not necessary, but ethical issues were discussed at each PDSA cycle with the project supervisor and practice manager.
Acknowledgments
We would like to thank Dr Sabrina Ong and Dr Bharati Shah as well the staff at the practice for their support and guidance throughout this QIP. We would also like to acknowledge Dr. Ann Wylie, Professor Anne Stephenson, and Dr Yvonne Batson-Wright for their continued support. This project has won prizes for ‘Best Oral presentation’ and ‘Most Sustainable Project’ at the GKT School of Medical Education’s QI Conference 2021 and the King’s Apothecaries prize 2021, and we would like to thank all involved in awarding and nominating this project.
References
Footnotes
Contributors NL, DZ and AN equally contributed to the writing of this paper as part of their Quality Improvement Project in year 4 placement in a General Practice setting. This team designed, implemented and measured the impacts of each PDSA cycle. In the writing of this paper, NL contributed to the Introduction, Methods and Results, AN contributed to the Results, data analysis and interpretation and DZ contributed to the Lessons and Limitations, Sustainability and Conclusion; collectively writing this manuscript. NL presented the paper at King's College London QIP conference 2021, winning ‘Most Sustainable’ and ‘Best Oral Presentation’. SBYO supervised the overall project and reviewed the manuscript. Practice staff assisted with EPR training and storage of confidential patient data. NL took up the position of guarantor for this study.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.