Article Text
Abstract
Background Proper sedation is integral to ensuring the safety and comfort of children on mechanical ventilation (MV). Sedation protocols help to achieve this goal and reduce the duration of MV. We have observed varied sedation approaches, sedation score targets and sedative use by our physicians, which were manifested as oversedation and undersedation with associated accidental extubation. Hence, we aimed to implement a standardised sedation protocol and assess its impact on mechanically ventilated paediatric patients.
Methods A multidisciplinary quality improvement team was formed to develop and implement a standardised sedation protocol for mechanically ventilated paediatric patients. COMFORT-Behaviour (COMFORT-B) Scale score was used to assess the sedation targets and define undersedation, oversedation or adequate sedation. Our goal was to achieve adequate sedation during 90% of the sedation period. Based on the model for improvement methodology, we used plan–do–study–act cycles to develop, test and implement the new sedation protocol.
Results There was an immediate percentage increase in COMFORT-B Scale scores within the target sedation level, which was associated with a gradual decrease in the need for intermittent sedation doses over sedation infusion in the preimplementation, improvement and control phases (6.3, 4.9 and 3.1 sedation doses/12 hours/patient, respectively) to achieve adequate sedation target.
Conclusions The standardisation of sedation protocols was safe and efficient, and improved the sedation quality in mechanically ventilated paediatric patients.
- patient safety
- critical care
- paediatrics
Data availability statement
Data are available upon reasonable request. The data underlying this article will be shared on reasonable request to the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Approximately 50% of critically ill infants and children in paediatric intensive care units (PICUs) require mechanical ventilation (MV). Sedation is initiated on these children to allow for tolerance of MV, improve synchronisation, avoid accidental extubation and reduce the associated physiological stress.1 Ensuring the safety and comfort of these children is integral to the practice of paediatric critical care.2 However, excessive sedation leads to prolonged MV and extended PICU and hospital length of stay (LOS), thus increasing the risk of ventilator-associated pneumonia, lung injury, delayed recovery, tolerance, withdrawal and delirium.3 4 Proper sedation, which is attainable and safe for children on ventilation, aims to ensure that they are calm, easily roused and can be readily evaluated.5 In critically ill adults, protocolised sedation reduces the incidence of ventilator-associated pneumonia and duration of MV.6–8 Evidence of reduction of the duration of MV due to protocolised sedation practices varies in paediatric populations; however, implementation of these protocols has proven feasible.9 Overall, there were various limitations in paediatric studies; for instance, although the study by Curley et al was a randomised controlled trial, it included only children with acute respiratory distress syndrome.5 10–14
Settings
Our quality improvement (QI) study was conducted at King Abdullah Specialised Children’s Hospital, which is a tertiary academic centre in Riyadh, Saudi Arabia, that has a 25-bed closed medical and surgical PICU, with approximately 1000 patients admitted per year. Our PICU patients are managed by multiple attending physicians and paediatric intensive care fellows and residents, some of whom belong to other specialties, such as paediatric emergency, anaesthesia and surgery, and are doing their rotation in PICU. Our PICU patients are served by a nursing staff at a 1:1 ratio. We have observed varied sedation approaches, different sedation score targets, and several instances of undersedation and oversedation. Oversedation may expose the patient to side effects and a longer duration of sedation. On the other hand, the lack of a standardised sedation protocol was an identified cause in undersedation associated with accidental extubation reported in our PICU. Therefore, we initiated this QI project, and a multidisciplinary QI team was created, consisting of two paediatric intensivists, a clinical pharmacist, a PICU nurse and a QI facilitator. The project aimed to implement a standardised sedation protocol and to assess its efficacy on mechanically ventilated paediatric patients in the PICU.
Methods
Baseline measurement
Several sedation scales have been established for critically ill patients. We defined the sedation level by the COMFORT-Behaviour (COMFORT-B) Scale, a validated sedation scale for mechanically ventilated children15 16 that includes six behavioural items: state of awakening, levels of agitation, spontaneous ventilation, characteristics of movements, muscular tone and facial tension. Scores of 6–10, 11–22 and 23–30 indicate excessive sedation, adequate sedation and insufficient sedation, respectively.15 16
Baseline data were collected prospectively over 3 months, from July to September 2017, by assigned PICU nurses. A simple audit tool was used for each patient from chart reviews in the hospital information system.
A non-procedural intermittent sedation dose was defined as a dose administered over an infusion to achieve adequate sedation, but not prior to a procedure such as a line insertion. The optimal sedation-level stability and sustainability were when achieved by sedation infusions that required less frequent use of non-procedural intermittent sedation doses. The bedside nurses assessed sedation levels by scoring the COMFORT-B Scale every 4 hours regularly and 30 min after each administration of intermittent sedation doses.
Patients aged 0–14 years who were admitted to the PICU and predicted to require MV for >24 hours were included. Patients having an infusion of neuromuscular blocking agents or undergoing sedation for medical treatment, such as seizure management, were excluded.
The completed preimplementation data included 38 patients and showed that only 51% of the sedation period was within the targeted COMFORT-B Scale sedation level (score of 11–22). Patients received an average of 6.3 non-procedural intermittent sedation doses per 12 hours, reflecting inadequate sedation. Thus, we planned to develop and implement a standardised sedation protocol for mechanically ventilated paediatric patients in the PICU to achieve adequate target sedation (COMFORT-B Scale scores of 11–22) during 90% of the sedation period.
Design
We formed a multidisciplinary QI team comprising two paediatric intensivists, a clinical pharmacist, a PICU nurse and a QI facilitator, with the goal of establishing a standardised protocol for sedation and monitoring the process and outcome of the said protocol. After baseline data analysis and review of literature and evidence supporting the safety and feasibility of a standardisation protocol, the team determined that establishing a standardised protocol for sedation may be the best way to achieve adequate sedation in patients, thus preventing the consequences of undersedation or oversedation. The comprehensive review of evidence regarding the assessment and management of paediatric sedation served as a guide in the protocol development. The defined process measures included the following: the number of non-procedural intermittent sedation doses administered to the patient to achieve the target comfort level, the total number of COMFORT-B Scale assessments performed by the nurse during a 12-hour shift and the rate of compliance to the sedation protocol by the physician. There were two outcome measures: percentage of scores achieving the target COMFORT-B levels related to the total scores and the total duration of sedation per patient (table 1). Physicians were considered non-compliant to the protocol if they did not specify the target sedation in their sedation order or if the sedation infusion increment/decrement in the sedation protocol was not followed. Consequently, the shift would be labelled as a non-compliant shift, except in situations wherein changes needed to be made due to the haemodynamic or respiratory stability of a patient.
Process measures and outcome measures for the sedation protocol
Improvement strategy
Based on the model for improvement methodology, we used the plan–do–study–act (PDSA) cycles to develop, test and implement the new sedation protocol. We executed three PDSA cycles over the project period of 2 years. A simple audit tool was used for data collection. A total of 38 patients were included in the 3 month period before protocol implementation. The project lasted for 24 months, and a total of 82 patients were included in the postimplementation group. Seven patients in the preimplementation period and 12 patients in the postimplementation period were excluded because they had an infusion of a neuromuscular blocking agent or sedation for seizure management.
PDSA 1, December 2017
Plan
Develop a standardised sedation protocol.
Pilot the protocol among PICU physicians and nursing staff.
Do
The PICU sedation protocol was developed by the QI team based on the best available practices.2 5 10 12 17 There were two sedation regimens: morphine with midazolam and fentanyl with midazolam. The initial regimen and sedation target (COMFORT-B Scale score) were determined by the physicians. The protocol also included intermittent doses, if needed, to achieve the target sedation. These intermittent sedation doses consisted of the same sedation infusion medication; that is, if fentanyl was running as an infusion, the intermittent dose would be fentanyl as well.
The sedation algorithm was developed and placed at the bedside of each patient to guide the treating teams.
The physicians and nursing staff participated in several 30 min educational sessions about the sedation protocol, which included presentations on the two sedation regimens in the protocol, sedation targets and sedation assessment using the COMFORT-B Scale. The sessions ended with interactive scenarios.
The clinical resource nurses (CRNs) also educated all PICU nurses on the use of the sedation protocol.
The nurse working group of the sedation team introduced the protocol to all nurses through training sessions. The nurse working group answered questions and explained the algorithm of sedation protocol to them.
Testing took place in September 2017 and lasted for 3 months.
Study
The compliance rate was 30%–40% after the initial implementation, which was considerably behind our target of 90% (figure 1).
Feedback from the nursing staff indicated that most orders were executed by residents who were unaware of the new protocol and only had their rotations in the PICU monthly.
Some physicians were not satisfied with the standardised protocol, according to feedback from the attending physicians.
Physicians’ compliance with the sedation protocol. PDSA, plan–do–study–act.
Act
The section head of the PICU and the QI team leader had a meeting with the PICU team and clarified their concerns. Subsequent meetings were planned to address the concerns of the team.
Residents had more involvement in protocol education through the monthly PICU orientation.
Based on the outcomes from PDSA cycle 1, we performed the second PDSA cycle as follows.
PDSA 2, July 2018
Plan
Paediatric intensivists of the QI team to conduct regular teaching sessions and disseminate the protocol.
Training on protocol and entry of the orders in the electronic medical record to be included in the monthly PICU orientation of the residents when their rotations start.
Continue monitoring compliance to the sedation protocol.
Do
The sedation standard order set was developed by the hospital information system.
The intensive care physician from the QI team conducted four educational sessions for the attending intensivists and fellows. The importance of sedation standardisation and compliance with the protocol regimen were the focus of these sessions.
The sedation protocol was added to the orientation manual of the rotating residents and was distributed at the beginning of their PICU rotation. It included information about sedation assessment and management and the use of the sedation protocol standard order in the hospital information system.
Study
The optimal sedation goals (set at 90%) within the adequate target sedation level were not achieved.
The compliance rate improved to 50%–60% (figure 1).
Act
A series of weekly, brief education sessions among the PICU physicians and nursing staff were conducted before daily patient rounds.
The CRNs tracked daily shift compliance. They provided real-time escalation to the multidisciplinary team through the daily PICU huddles if issues were noted.
We reviewed the progress of the current project and monthly sharing compliance data in the PICU multidisciplinary division meeting.
Based on what we learnt from PDSA cycle 2, we performed the third PDSA cycle as follows.
PDSA 3, March 2019
Plan
Improve communication among PICU nurses and physicians regarding the sedation management and plan sedation target and intermittent sedative requirements per shift.
Initiate root cause analysis during PICU division meetings.
Include project progress as a standing agenda in PICU multidisciplinary monthly meetings.
Do
An escalation process was implemented for when the sedation protocol had been breached. The nursing staff was educated regarding these escalation steps and how to raise concerns related to sedation status to keep nurses and PICU physicians accountable.
Sedation protocol compliance was added as a regular item in the PICU daily huddles, wherein nurses and physicians discussed the challenges and solutions to such.
Study
Root cause analysis revealed that on their daily rounds, bedside nurses did not report patient sedation scores, target sedation and intermittent non-procedural sedation doses administered during the previous shift.
The sedation QI team agreed that the daily sedation report of the nurses would improve physician compliance and emphasise the importance of sedation target achievement.
Act
All PICU nurses (n=110) underwent reorientation. General reviews were made regarding sedation, analgesia and evaluation with COMFORT-B scale. Protocol algorithm and clinical scenarios were discussed to illustrate protocol use and necessary escalation steps.
Patient and public involvement
There was no patient and public involvement in the design or dissemination plans of our research.
Results
With the downward trend of intermittent sedation doses, the results showed an immediate percentage increase in COMFORT-B Scale scores within the target sedation level for each patient on MV after protocol implementation, especially after PDSAs. The percentage in COMFORT-B scores reached 75%–85% in the last 3 months of the project (September–December 2019), which improved from the preimplementation (September–December 2017) percentage of 40%, thus indicating that patients had improved sedation levels and less frequent intermittent sedation doses (figure 2).
Percentage number of COMFORT-Behaviour Scale assessment within the target adequate sedation level. PDSA, plan–do–study–act.
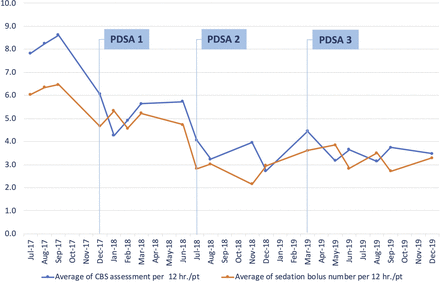
On observation of the process changes over 24 months, a gradual decrease in the average doses of intermittent sedation in the preimplementation, improvement phase and control phase (6.3, 4.9 and 3.1 sedation doses/12 hours/patient, respectively) was revealed. As shown in figure 3, this decrease was also associated with a reduction in the average number of COMFORT-B Scale assessments, decreasing from 8.2 times per 12 hours/patient in the preimplementation phase to 6.0 times per 12 hours/patient in the postimplementation phase. Eventually, it decreased to 3.5 times per 12 hours/patient after the PDSAs.
Average number of intermittent sedation doses per 12 hours/patient and average number of CBS assessments per 12 hours/patient. CBS, COMFORT-Behaviour Scale; PDSA, plan–do–study–act.
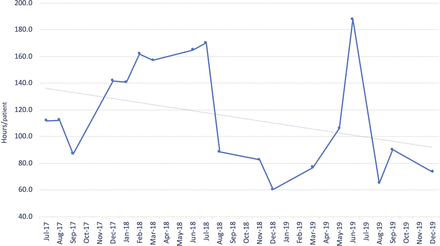
The mean duration of total sedation hours decreased over the course of the project (figure 4).
Mean of sedation period (in hours).
Discussion
Statement of principal findings
Implementing a sedation protocol through this QI project resulted in better sedation practices for intubated patients in our PICU, as reflected by the reduction in the duration of sedation and the number of intermittent non-procedural sedation doses. The latter was reduced from an average of six to seven doses/12 hours to three to four doses/12 hours, reflecting a stable sedation course with fewer fluctuations.
Interpretation within the context of a wider literature
Despite the low quality of evidence supporting the effectiveness of sedation protocols and guideline implementation in mechanically ventilated paediatric patients, some studies have shown the positive impact of these protocols on patient outcomes, which was consistent with our results.5–8 In a pilot study that used a pre–post design, Keogh et al showed that sedation guidelines were feasible and acceptable in clinical practice as these positively impacted the sedation duration and dosage. However, no effects on ventilation duration or LOS were noted.18 A systematic review by Poh et al suggested that the use of sedation protocols was associated with a reduction in unplanned extubation, drug withdrawal, sedation duration, sedation dose, and PICU LOS.11 Likewise, in our study, we could reduce sedation duration and the number of intermittent sedation doses. Most of the improvements in the COMFORT-B Scale scores reached the target and reductions in sedation doses occurred during the early phase of the improvement process (after PDSA cycles 1 and 2), as shown in figures 2 and 3. However, another intervention (PDSA cycle 3) was implemented to enhance nurse–physician communication to prevent any undesired process shifts due to a lack of proper communication. Despite its feasibility, implementing a sedation protocol in critically ill children was a significant shift in our PICU workflow and daily practices. A stepwise strategy with gradual changes in roles and responsibilities was executed. While it was the responsibility of the PICU physicians to choose the appropriate sedation regimen and initiate and change drug infusion rates, the nurses were allowed to give extra sedation doses as they deemed necessary. Escalation to the physicians was required if the sedation target was not achieved and an infusion rate change was considered. The successful implementation of the protocolised sedation guidelines resulted in better comfort provided to PICU patients and a decreased number of COMFORT-B Scale assessments, encouraging our nurses to lead sedation and analgesia practices through collaborative nurse-driven sedation protocols in the future. Different protocols and practices described in the literature have prevented critical care societies from developing an international consensus on the best sedation practices.11 However, we used the most common practice in our unit and developed a two-arm sedation protocol: fentanyl–midazolam and morphine–midazolam to avoid possible errors related to unfamiliarity with other drugs or protocols. Many challenges were encountered with this practice change. The various levels of experience and confidence of the nurses required multiple educational sessions to ensure their competency and readiness for each step, which led to implementation delays. The rotating residents and non-PICU fellows also required further attention to ensure their compliance with the new sedation protocol. Treatment of complex cases and critically ill patients who were hemodynamically unstable was challenging, as nurses found it difficult to make decisions regarding sedation and analgesia. Therefore, a multidisciplinary approach involving physician–nurse collaboration with a clear escalation process was necessary to provide the needed support and enhance the confidence of the nursing staff.
Implications for policy, practice and research
Promoting clear pathways for communication and escalation is key to the successful implementation of a new practice as these minimise the variation between team members. Regular periodic assessment of the process was challenging due to the lack of manpower in this QI project. However, it was essential in maintaining the correct direction and in detecting any deviation from the intended pathway. Furthermore, the sedation standardisation project data, including process and outcome measures, were presented regularly in the PICU dashboard, which is an electronic board located in the PICU nurse station that presents data for quality projects alongside other key performance indicators in our PICU. Regular data sharing with the PICU staff (physicians, nurses, pharmacists and trainees) was fundamental for successful implementation as it provided continuous stakeholder support and clear insights into the achievements and challenges faced by the PICU team.
The standard order set in the hospital information system and the creation of a policy and procedure of sedation improved the compliance of physicians and the sustainability of the programme over 2 years. The medical and pharmacy teams developed a protocol based on the best available evidence and practices, while the nursing staff ensured protocol feasibility and ease of compliance during implementation. Daily huddles and PICU division meetings were powerful engagement tools for communicating project progress.
Strengths and limitations
We have demonstrated that a paediatric sedation protocol improved the comfort of patients, achieved a more stable sedation level, and led to fewer intermittent sedation doses and comfort assessments, thus decreasing the workload of the nursing staff. By including the sedation protocol in the PICU order set through the health information system, it became routine practice in the PICU. Furthermore, we plan for collaborative nurse-driven sedation protocols in the future to ensure the sustainability of the protocol and to encourage our nurses more. Meanwhile, our plan consists of regular quarterly monitoring of outcome measures and their appropriate interventions.
This study did not consider any patient clinical outcomes, such as MV-free days, PICU LOS and unplanned extubation, which may be affected by changes in sedation practices or other factors. Thus, this is one limitation of the study. Furthermore, although data from our study suggested that a paediatric sedation protocol likely improved sedation quality and decreased sedation duration, this cannot be attributed to protocolised sedation practice alone. Other factors such as MV practices, type and severity of illness must be considered in future studies to accurately determine the relationship between sedation protocol and outcome. However, it is important to note that there were no major changes in MV management strategies or other factors, such as the type of patients and their severity of illness, before or during the implementation period.
Applied interventions in this QI project such as a two-armed sedation protocol in a single-centre, medical–surgical, non-cardiac PICU might not be generalisable to other PICUs due to differences in the type of patients (paediatric cardiac ICUs), allocation of resources (eg, nurse:patient ratio), or variability in sedation and analgesia practices.
Lastly, the experience and expertise of the physicians cannot be excluded as the cause of the improvement.
Conclusions
Having a sedation protocol for mechanically ventilated paediatric patients is safe, efficient and improved sedation quality. A multidisciplinary team and stakeholder involvement with regular process assessments are essential for successful implementation. The effects of protocolised sedation practices on withdrawal, delirium incidence and other patient outcomes need further exploration.
Data availability statement
Data are available upon reasonable request. The data underlying this article will be shared on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
References
Footnotes
Twitter @TarekHazwani
Contributors TH: substantial contributions to the conception, and was responsible for study design and oversight, data collection, data analysis, and drafting the manuscript. AAA: substantial contributions to the conception, data collection, data analysis. YK: substantial contributions to the conception, data analysis, and writing processes. AAS: substantial contributions to the conception, involved in study procedures and implementation of the protocol. SAE: substantial contributions to the conception and involved in study procedures and implementation of the protocol, and data collection. HA: substantial contributions to the conception, data analysis, and writing processes.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.