Article Text
Abstract
Background At present, there are no validated quantitative scales available to measure patient-centred quality of care in health facilities providing services for tuberculosis (TB) patients in India and low-income and middle-income countries.
Methods Initial themes and items reflective of TB patient’s perceived quality of care were developed using qualitative interviews. Content adequacy of the items were ascertained through Content validity Index (CVI) and content validity ratio (CVR). Pilot testing of the questionnaire for assessing validity and reliability was undertaken among 714 patients with TB. Sampling adequacy and sphericity were tested by Kaiser-Meyer-Olkin and Bartlett’s test, respectively. Exploratory and confirmatory factor analysis was undertaken to test validity. Cronbach’s α and test–retest scores were used to test reliability.
Results A 32-item tool measuring patient-perceived quality of TB distributed across five domains was developed initially based on a CVI and CVR cut-off score of 0.78 and cognitive interviews with patients with TB. Bartlett’s test results showed a strong significance f (χ2=3756 and p<0.001) and Kaiser-Meyer-Olkin was measured to be 0.698 highlighting data adequacy and correlation between the variables. Exploratory factor analysis with varimax rotation extracted 4 factors related to 14 items with Eigen values >1 which accounted for 60.9% of the total variance of items. Correlation (z-value >1.96) between items and factors was highly significant and Cronbach’s α was acceptable for the global scale (0.76) for the four factors. Intraclass correlation coefficient and the test retest scores for four factors were (<0.001) significant.
Conclusion We validated a measurement tool for patient-perceived quality of care for TB (PPQCTB) which measured the patient’s satisfaction with healthcare provider and services. PPQCTB tool could enrich quality of care evaluation frameworks for TB health services in India.
- quality measurement
- patient satisfaction
- patient-centred care
- health services research
Data availability statement
Data are available on reasonable request. Data are available on reasonable request from the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Quality of care provided for patients with tuberculosis (TB) in public and private health facilities are considered important.
WHAT THIS STUDY ADDS
There are no validated tools available at present to measure quality of TB care from the patients perspectives.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
We validated a tool to measure the patient-perceived quality of TB care. This tool could be used for evaluating quality care standards for TB in India
Introduction
Tuberculosis (TB) is a preventable and completely curable disease, but still millions of deaths occur due to TB in the low-income and middle-income countries (LMICs) due to the poor-quality care provided for patients with TB.1 While access to TB services has been scaled up considerably, still the quality of care remains an important factor.2 The importance of quality of care for patients with TB in both public and private health facilities remains an important determining factor in expanding standard diagnosis and treatment coverage for TB in India, which suffers a quarter of the global TB burden.3 4
The success of TB control programmes depends critically on the healthcare settings and the quality of care available there for the patients.5 While the quality of care is considered vital in TB healthcare settings, still the patient perspective in terms of care is either unmeasured or not measured properly. Patient experiences in health facilities remains rarely focused and assessed in LMICs health systems and there is a need to evolve valid indicators and measurements in this context. A recent systematic review study concerning TB patient’s user experience and their satisfaction with the healthcare found that there was considerable variation in measuring such experiences and satisfaction. The review emphasises that there is a lack of a standardised patient-centred tool for use in TB healthcare settings which need to be developed for better implementation of patient-centric TB services in LMICs.6
Considering patient-centred care is crucial to achieving favourable health outcomes for patients with TB, it is imperative to have a standardised quantitative tool that is valid and reliable to capture the experiences and satisfaction of patients with TB across different healthcare settings.7 In this background, we attempted to develop and test a quantitative tool to assess the TB patient-perceived quality of care provided in both public and public–private mix (PPM) facilities in an Indian setting with a high burden of TB.
Methodology
Study design
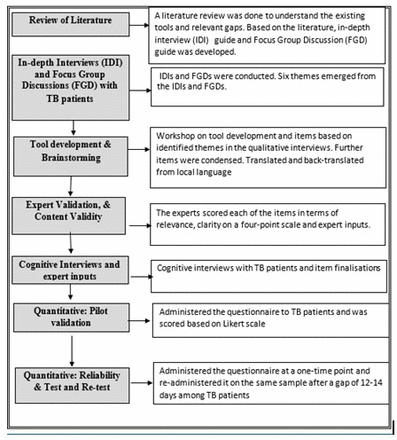
The study used qualitative and quantitative methods in a phased manner (figure 1).
A step-by-step description of the study process for developing and validating a patient centred quality of care tool for TB. TB, tuberculosis.
Study settings
The study was conducted in between 2018 and 2020 in Chennai, a metropolitan city of Tamil Nadu state in India with a population of almost eight million. Chennai suffers a disproportionate burden of TB, contributing to 14 370 (13.7%) of the total cases of TB in the state as on 2019.8 TB burden within Chennai is also found to be disproportionately affecting the urban slum population which comprise 28.89% of the total population of Chennai. The National TB Elimination Programme (NTEP) of Chennai is a publicly funded programme that operates through its 36 treatment units, 71 designated microscopic centres to provide free diagnostic and TB treatments services to the patients.9 Of the total patients with TB in Chennai only 50% are notified in the public sector and remaining in the private sector.10 Chennai is a setting where there is a considerable transfer of patients from the public to the private sector and vice versa.11 The sample catchment area of this study encompasses all the 36 TB treatment units in 15 zones of Chennai Corporation.
Study phases
Review of literature and formative phase
A review of the literature was undertaken and prior work on the subject of patient’s perception of quality of care was assessed in TB context. The information obtained from the literature review were used to develop semistructured qualitative interview (SSI) and focus group discussion (FGD) guides. The participants for the qualitative phase were patients with TB above 18 years of age and who were on anti-TB treatment for at least 2 months from both PPM healthcare settings in Chennai city. Trained investigators used the guides to conduct IDIs and FGDs as per the standards of qualitative study.12 A total of 72 in-depth interviews were conducted which lasted for 30–45 min and were audiorecorded. The participation of patients in IDIs was such that they received treatment services from public, PPM and/or both. (online supplemental table 1). Ten FGDs were conducted, in which a total of 78 patients with TB participated from both the PPM healthcare facilities. SSI and FGD guides included probes that were particularly aimed at understanding the reasons and pathways through which the patients have navigated between various public and private health facilities for TB. The qualitative interviews explored on the ‘experiences’ and ‘information’ and ‘perception’ which have influenced the patients to switch over between the public and PPM healthcare facilities. The interview guide was pilot-tested with a few patients under both public and PPM facilities. The guide was updated to include new probes generated from the pilot interview. The order of the probes was reorganised to facilitate a logical flow of inquiry and to get a sense of the ‘pathway’ through which the patient has navigated the health facilities during her treatment period.
Supplemental material
The IDIs and FGDs were recorded and were further transcribed and coded using a thematic approach and analysed using Dedoose software. Content analysis methods was used for analysing the data without a preconceived theoretical framework. These themes were used to construct categories and illustrative quotes from the data. Based on the literature search and the themes that emerged from the interviews, questions in the context of patient-centric care in TB treatment facilities were developed. A total of 64 items (questions) were framed under the stated themes. Subsequently, a series of brainstorming sessions among the researchers led to the elimination of repetitious questions or questions that measured similar components.
Quantitative phase
Content adequacy assessment through expert validation
Findings from the qualitative phase were used to synthesise and list items (questions) which were further subjected to expert validation for their relevance and clarity. The expert committee was diverse and inclusive of experts from the field of social science, statistics, and clinical medicine. The committee also included front-line health workers and doctors from the NTEP. For this purpose, a content validation form was created that defined the construct and allowed experts to provide feedback on the clarity and relevance of each of the items. Panel members were asked to rate instrument items in terms of clarity and their relevancy to the instrument on a 4-point ordinal scale (1 (not relevant), 2 (somewhat relevant), 3 (quite relevant), 4 (highly relevant)). A table was added to guide experts for the scoring method (online supplemental table 2).
To obtain a Content Validity Index (CVI) for relevancy and clarity of each item, I-CVI which captures the proportion of agreement on the relevancy of each item between 0 and 1 was considered. The content validity ratio (CVR) was used to assess whether the content is appropriate for that instrument which was calculated using the formula CVR= (Ne − N/2)/ (N/2), in which the Ne is the number of experts indicating the content as ‘essential’ and N is the total number of experts.
In addition to collecting quantitative data, the experts were provided with an opportunity to provide free-text comments. The experts suggested translation changes, the flow of the questionnaire and the number of questions to be added under each domain. Thus, the quantitative data and qualitative expert inputs were utilised to improve the content validity of the tool thereby to ensure its better performance. Standard acceptable score for the I-CVI considered for this study was 0.78.13 14 After expert validation, cognitive interviews were conducted among patients with TB (n=26) from both public and PPM sectors to ensure that the content and language of the questions were comfortable from the patients perspective and their responses were appropriate towards these questions.
Validity testing
As the next step, pilot testing of the questionnaire for assessing validity of the tools was undertaken. The target population (patients with TB) for the developed tool were interviewed for pilot testing of the tool. The data were collected from adequate sample size to appropriately conduct subsequent analyses. The number of variables or items to be assessed was used to calculate the sample size needed to obtain robust results. An item-to-response ratio of 1:20 was adopted in this study. The minimum sample size of 20 individuals was considered for each item in the tool and thus for the total 32 items, a minimum sample of 640 was considered. However, we enrolled 714 patients with TB, which were more than the minimum sample size. The respondents were selected using systematic sampling from all 99 Revised National Tuberculosis Control Program (RNTCP) centres and 26 PPM centres in Chennai, (10). The respondents were selected based on the inclusion criteria: that the patient must (1) have consented to participate in the quantitative assessment, (2) be above the age of 18 years, (3) have been registered and taking treatment for at least 2 months in RNTCP and PPM. The respondents were asked to measure the 32 items on a four-point Likert Scale, where satisfaction level ranged from 1—extremely dissatisfied to 4—extremely satisfied, frequency of experiences ranged from 1—never to 4—always, facility rating ranged from 1—not available to 4—good and the responses were collected and analysed further
Reliability testing
To ensure internal consistency, reliability was estimated to assess how well the items that reflect the same domain yield similar results. The test and retest reliability of the instrument was assessed by administering the questionnaire at a time point and readministering it on the same sample after a gap of 12–14 days.15 16 Based on the assumptions of population reliability value as 0.50, expected reliability of the tested tool to be 0.75 with the power of 80% and 95% CI with two replicates and 20% lost to follow-up. The sample size for this test–retest reliability was calculated to be 54 patients with TB. The data were collected from 48 randomly selected patients with TB for test–retest reliability who were found eligible during the study period.
Statistical analysis
Descriptive statistics were generated to assess the distribution of variables and exploratory factor analysis (EFA) was used to explore and identify meaningful item clusters by data reduction. A principal axis method was used which accounted for common, specific and random error variances. We used two tests to determine if the data are suitable for factor analysis: Bartlett’s and KMO. Kaiser-Meyer-Olkin test and Bartlett’s test were used for testing sampling adequacy and sphericity, respectively.17 Eigenvalues greater than 1 (Kaiser criterion) was used to determine the number of factors to retain. The objective of EFA was to identify items that load on a single factor or domain. Pattern loadings of 0.40 or higher were used as criteria to include items. Confirmatory factor analysis (CFA) was used to determine the number of factors required in the data and to decide on the relation between the items’ and factors.
To assess test–retest reliability, item-total correlations were calculated. Internal consistency for reliability was established using Cronbach’s α. Values of α 0.80 or higher were considered acceptable for adequate internal reliability. Intraclass correlation coefficients were calculated on subscale and total scores. Intraclass correlations were used to assess inter-rater agreement between pairs of scores provided by two different raters.17 The quantitative data collected were analysed using STATA V.16.0. Statistical significance was determined at 5%.
Results
Findings from qualitative phase
Domains of quality of care from patient’s perspective
Thematic content analysis of data obtained from patients with TB attending public and PPMs through IDIs and FGDs resulted in the identification of five major themes or domains of quality of care for TB. These five major themes included factors related to TB services in the facilities, attitude of healthcare providers towards patients with TB, content, style and type of information provided for the patient with TB in the facility, basic amenities available and expected by patients with TB in the health facilities and the affordability of TB treatment in the facility. We found these five domains were consistently emerging across all the TB patient interviews in both the setting and also for patients with TB who switched between the facilities. Based on these major domains a total of 64 questions were framed. Subsequently, a series of brainstorming sessions among the research team was conducted and led to elimination of repetitious questions or questions that measured similar components and resulted in 32 items and it was further translated into the local language and was back-translated to English
Expert validation and cognitive interviews
The results of the expert validation of 32 items on the items’ relevance and clarity are provided in (online supplemental table 3). The results show that a total of eight items had a CVI and content validation ratio (CVR) score of less than 0.78 which were excluded. Further based on the cognitive interviews conducted among 26 patients with TB and based on the inputs received, few items were further added and further modified. The final questionnaire consisted of 32 items (table 1).
Finalised domains and 32 items based on qualitative findings, content validation and cognitive interviews
Findings from quantitative pilot validation
Demographic profile
The demographic profile of the respondents (n=714) to whom the tool was administered for assessing internal consistency is presented in online supplemental table 4.
Exploratory factor analysis
EFA with varimax rotation was performed to explore the responses of sampled patients with TB 32 times. Concerning data adequacy and correlation between the variables Bartlett’s test results showed a strong significance f (χ2=3756 and p<0.001) and Kaiser-Meyer-Olkin was measured to be 0.698, which was above the required 0.5. These tests highlighted the substantial correlation in the data and appropriateness for factor Analysis.
Online supplemental table 5 shows the principal factor analysis based on extraction of items under each factor based on their values. Further EFA varimax rotation extracted four factors with eigen values >1 accounted for 60.9% of the total variance (table 2 and online supplemental figure 1). Of this, factor 1 made unique contribution to the variance of items 12, 15 and 16 which were related to the doctor’s care and thus was labelled ‘Satisfaction with Doctor’s Care’. Factor 2 made unique contribution to the variance of items: 17, 18 and 19 which were related to the patient’s information, and thus was labelled as ‘Satisfaction with the information given by the healthcare provider’. Factor 3 contributed to the variance of the items 8, 9, 10 and 13 which were related to the health visitor and was labelled as ‘Satisfaction with the health visitor’. Factor 4 contributed to the variance of four items: (1, 2, 3 and 4) related to TB services at the facility and hence labelled as ‘Satisfaction with the TB services’.
Total variance explained by eigenvalue against the number of factors
Confirmatory factor analysis
CFA was undertaken based on the factors extracted in EFA and were found to be confirmatory (table 3) of the results of EFA. The factor loading for most of the items was above 0.60, highlighting the agreement between latent factors) and the observational items. The root mean square error of approximation equalled 0.08, showing an acceptable fit. It was found that correlation between items and factors were also significant (z value >1.96).
Confirmatory factor analysis to assess factor loading
Reliability of items
Item-total correlations ranged from 0.82 to 0.93 for factor 1, from 0.79 to 0.86 for factor 2, and from 0.65 to 0.76 for factor 3. Except for factor 4, the correlations ranged from 0.65 to 0.77 for factor 4 and from 0.40 to 0.76 on a global scale. Even factor 4, with a modest correlation of 0.26 was justified retention based on the global scale of 0.559. Item-total correlations were considerably strong and highlighted that items belonging to the same factor measured the same construct. The reliability of the scale was represented by using the Cronbach’s α coefficient. The reliability of the Cronbach’s α was acceptable for the global scale (0.76) or higher as evidence of adequate internal reliability (table 4).
Cronbach’s alpha for the factors and the global scale
Test–retest reliability
The ‘test’ ‘retest’ scores at 2 weeks were correlated among 48 patients. Findings show the intraclass coefficient that emerged from our analysis, including the overall score, were significant (<0.001), indicating a strong test–retest reliability (online supplemental table 6).
Discussion
While access to TB services in India has been scaled up considerably, still the quality of care remains an important gap as evidenced by a series of studies conducted in the LMICs.3 18 The importance of quality of care for TB in public and private health settings remains a determining factor in expanding standard diagnosis and treatment coverage for TB. A systematic review on the international and national standards of TB care followed in Indian TB care settings identified only one standard which was measurable of patient-centric care.19
In this context, we undertook an attempt for pilot validation of a measurement tool to assess the TB patient-perceived quality of care in public and private healthcare facilities in a resource-poor setting. We developed a 32-item scale of which fourteen items loaded in four unique factors which contributed to a 60.0% variance of items. These four factors were all found to be related to the care-seeking process of patient with TB in the facility which has been missing in the conventional input and output based TB quality of care evaluation frameworks.5 We named these twelve items as patient-perceived quality of care for TB (PPQCTB).
The validated PPQCTB tool underscores that the satisfaction of the patients with TB concerning the physician care and services of healthcare providers to be important from their perspective. The identified factors help explain and address why a significant proportion of patients are loss or dropped constantly throughout the TB cascade of care in Indian public and private health facilities.20 The perceived role of a physician and the healthcare provider in meeting the quality of care demands of patients identified in our study corroborates with the quality of care factors—like lack of choices for patients to develop long term trust with doctors- associated with primary healthcare services in LMICs, thus adding value to our developed tool.21
The four factors with high loading were found to be statistically valid and reliable, as shown by eigenvalues and high Cronbach’s α. CFA results highlight that there is less overlap between the four factors and were found to represent separate constructs of patient’s perceived care. Item-total correlations of the items demonstrated the reliability of the factor. Test–retest reliability measures test also showed consistency of the scores over time. These statistically valid results suggest that the patient-perceived quality of care is itself a comprehensive and quantitatively measureable construct and is not limited only to the medication adherence aspects of patients with TB in health facilities.6
While there are no prior studies using patient perception driven quality of care tools, in India or LMICs, our development of the valid 14-item measure is important as it is based on a primary study done among patients and other stakeholders.6 The tool holds intrinsic value when compared with ‘standard patient’ driven methods which are adopted in some settings.22 Our tested scale is also brief and simple which permits its wider adaptation and use in all public and private healthcare facilities.
A recent review of the indicators of the quality of TB care found that most indicators currently used emphasise on the input indicators pertaining to laboratory equipment’s, drug availability, human resources, adherence to protocol, etc.23 Patients experiences during the care cascade, their satisfaction level with providers attitude, communication style, privacy, time spent, etc, are not given attention in the quality of care context.5 Suboptimal or poor quality of care is a significant public health problem that can have negative impacts canon the treatment outcomes of patients with TB. There are considerable evidence which have assessed the positive impact of patient-centred care on clinical outcomes for patients.5 24 25 In addition, lack of patient-centred care is known to be associated with increased technological intervention due to the lack of connection between patients need and healthcare providers and this leads to increased resource utilisation,. This again increases healthcare spending in LMICs and again likely to lower quality of care for patients with TB.26 27
Thus in this context, the present validated PPQCTB tool provides the opportunity to measure the patient-centred care on a large scale across public and private. The use of this validated patient centres quality of care tool would help evaluate quality of care interventions for patients with TB and also will help improve long-term treatment and economic outcomes for the patients and the TB control programmes.
Strengths of the tool
The PPQCTB tool remains important from two different aspects. The tool was developed based on an iterative and participatory process that involved the patients with TB and other stakeholders who hold a key role in delivering the quality of care of TB provided. The representative sample which was used to develop and validate this tool comprise of patients and providers and experts at all levels of the care process in both public and private facilities. Such an inclusive and broad-based participatory approach has provided an intrinsic value and inputs to the tool which is important and often missed in the usual tool development process in the TB context. Our study also provided explanation and measurable indicators on the quality of care issues underlying the switch over of patients with TB from public to private facilities and vice versa which has been documented in earlier studies conducted in the same settings.9
Limitations
The proposed tool was developed based on the patient’s experiences and inputs from a metropolitan setting in India. Thus the patient-perceived factors and measures identified and validated may not be generalisable completely for TB care settings in rural or tribal settings of India. The use of this tool has to be yet tested for it impact on the patient treatment outcomes. This tool should be tested at other locations and adapted to fit the needs of other settings. Also, in addition, there is a need to assess which behaviours and characteristics of patients with TB are associated with patient perceived quality of care.
Conclusion
The development and validation of a fourteen item PPQCTB tool from the perspectives of the patient show that the measures are clearly distinguishable from the international standards of care for TB, followed by the WHO and NTEP in India. Of the 18 standards laid out by WHO, only 1 standard is related to patient-centric care with a narrowed reference towards treatment supervision for them. As a valuable addition to these standards of care, our PPQCTB tool has been developed by gaining insights from the actual patients who access a wide variety of facilities in a resource-poor setting. The tool has been benefited from its rigorous and iterative process of development and representativeness of sampling which makes it of direct relevance for the patients during the treatment processes. This unique patient-centric tool can facilitate the measurement of the quality of TB care services from the patient’s perspective both in private and public facilities. This tool could help strengthen the quality of TB care services and enable the emergence of a more patient-centred approach.
Data availability statement
Data are available on reasonable request. Data are available on reasonable request from the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Institutional Ethics Committee of the National Institute for Research in Tuberculosis (NIRT), Indian Council of Medical Research (ICMR), Chennai (IEC No: 2018033). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors would like to acknowledge the study participants and the NTEP staff for their willingness to participate in the study and the time spent for the quantitative and qualitative interviews. We thank The Commissioner, Greater Chennai Corporation for the support provided for the study. We acknowledge the knowledge and inputs given by the Social Scientist, Doctors, Medical Social Workers, academicians and tool development expert Dr. Shuba Kumar, from SAMRATH for the expert validity. We would like to acknowledge the project staff Ms. Angeline Miriam, Mr. Vinoth Babu and department staff Mr S. Kalaiselvi and Mrs Sentharo Ovung.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Concept, designing and methodology: MP, KN, BET, BW; Data collection, Analysis, management: BW, SR, DA, SA; Drafting of the manuscript: KN, MP, SR, TT, SGN; Tool development & validation: MP, BET, DA, VKJ, BW; Administrative approvals & funding acquisition: LJ, JM, MP, BET, RA; Manuscript revision and finalization: KN, MP, KN, BET, TT, SGN. KN will be responsible for the overall content as the guarantor.
Funding The study was supported by United States Agency for International Development (USAID) through Tuberculosis Health Action and Learning initiative (THALI) grant/ STOP TB Partnership/ United Nations Office for Project Services (UNOPS) under the TB free Chennai initiative (UNOPS/400/401/2017/00052).
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.