Article Text
Abstract
Polypharmacy, the concurrent use of multiple medications by one individual is a growing global issue driven by an ageing population and increasing prevalence of multi-morbidity[1]. Polypharmacy can be problematic: interactions between medications, reduced adherence to medication, burden of medication to patients, administration time, increased risk of errors and increased cost. Quality improvement methods were applied to identify and highlight polypharmacy patients with the aim of reducing their average number of regular tablets/capsules per day by 25%.
The project was delivered within a UK based 27 bedded hospice inpatient unit. A series of PDSA cycles studied interventions focusing on the identification of patients with polypharmacy, the highlighting of these patients to prescribers for review and the views of patients about their medication. For the purposes of the study, polypharmacy was defined as greater than ten regular medicines and/or greater than twenty regular tablets/capsules each day. The interventions tested included patients on regular paracetamol and strong opioids being offered a trial without regular paracetamol, a constipation guide promoting the use of combination laxatives, education of prescribers around dose strengths, checklist of recommendations was placed in case notes and a sticker was used on the medicine chart to highlight patients in need of polypharmacy review.
The introduction of a trial without paracetamol and a laxative guide led to reductions in polypharmacy. The sticker and checklist were successful interventions for highlighting patients with polypharmacy.
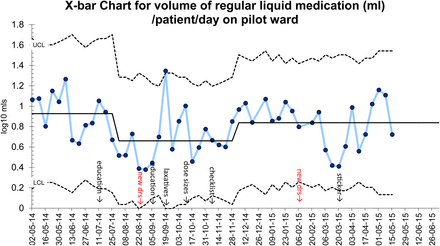
Quality improvement methods were used to plan, try, test and implement simple interventions for patients on the hospice inpatient unit. This has led to a 25% reduction in the average regular tablet/capsules burden , a 16% reduction in the average number of regular medications and a 30% reduction in the average volume of liquid medication per patient without an increase in the use of ‘as required’ medication or length of stay.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See:
Statistics from Altmetric.com
Problem
Polypharmacy, the concurrent use of multiple medications by one individual is an increasing global issue driven by an ageing population and increasing prevalence of multi-morbidity.1 The King's Fund Report 2013 ‘Polypharmacy and medicines optimisation: Making it safe and sound’ highlighted that the problem was a national priority. The prevalence of polypharmacy in a hospice in-patient setting has been examined previously2 ,3 and in line with national trends has increased over the last 12 years.
St Ann's Hospice offers specialist palliative care to adults with life limiting illness in the Manchester area, UK. In 2014, 325 patients were admitted to the Heald Green site where there are 27 beds. Consultant led care is provided on two wards. 31% of the patients admitted to the ward were subsequently discharged either to their home or a nursing home. Average length of stay was 22 days. In 2014, patients discharged from St Ann's Hospice in-patient unit at Heald Green were on an average of 9.5 regular medicines per patient per day, 15 regular tablets/capsules per patient per day and 55 millilitres (ml) of regular liquid per patient per day. Patients who died at the hospice were still taking an average of 11 regular tablets/capsules and 75ml of regular liquid medications a day, one week prior to their death (unpublished observations).
The aim of the project was to reduce the average number of regular tablets/capsules taken by patients on the ward (14 beds responsible to one consultant) by 25% over a period of 9 months (September 2014 to May 2015). This was initiated and led by a prescriber and pharmacist who felt polypharmacy was becoming an issue on the ward. Could strategies be developed to achieve this reduction for patients, without worsening their symptoms, increasing the volume of regular liquid medications taken or causing an increased length of stay? An additional focus was to gauge patients' views on the medication that they were taking.4
Background
It is known that the risk of medicine interactions increase with the number of medicines5 and that medicine handling alters with change of weight and metabolism at end of life. Adherence to a medication regime also decreases with increasing numbers of medication and it is recognised that the number of medication is burdensome for the patient and carers.6 Time spent on administering medication is considerable and detracts from other aspects of nursing care. More time is also required by the pharmacist and prescribers to check medicine charts. There is an increased risk of error with increased number of medications which then leads to time spent reporting errors and dealing with complaints and litigation. The cost of the medicines are important to consider but are probably surpassed by the costs of dealing with the complications of medication interactions with admission to healthcare facilities and prolonged length of stay.7 ,8 The ‘cost’ of this for a patient with a limited life expectancy should not be underestimated.
There are a number of effective approaches to reducing polypharmacy in geriatric medicine9–11 however there does not appear to be any approaches specific to hospice in-patient settings. It should also be noted that many of the medications highlighted as inappropriate in elderly care are commonly prescribed in palliative medicine.
Baseline measurement
In relation to the aim, data was collected from all patients who were on one ward, responsible to one consultant (potential maximum of 14 patients). This was done on a fixed day of the week after the consultant ward round using a data collection sheet. For each patient the number of regular medicines, number of regular tablets/capsules and volume of liquid (ml) medication (based on a best count using the British National Formulary [BNF] dose strengths) was recorded. The number of ‘as required’ controlled drugs used in that 24 hour period was also recorded; this was altered during the study period to include all ‘as required’ medication administered on the pilot ward. If a patient was discharged or died on the data collection day, their data was excluded as it would be incomplete. Counts were based on medication prescribed rather than medication actually taken. Where a range was prescribed the actual dose taken was used for analysis, if the medication was not taken then the previous dose taken was used. If a range was prescribed that had not been taken previously then the lowest dose in the range was used for calculations. A decision was made not to include saline nebules, emollients, nutritional drinks, or thickeners, as these are products that are often used, but not necessarily prescribed.
From this data an average of all parameters per patient on the ward was calculated.
In order to give a baseline, weekly point prevalence data was collected for patients in 14 beds (121 data points) over 10 weeks. It showed that inpatients at the hospice were taking an average of 8.9 regular medicines, 11.3 regular daily tablets / capsules and 49.4ml of regular liquid medicines each day.
A number of different interventions were planned that the authors thought would help reduce polypharmacy and the effect on the weekly data was observed. U charts12 were used to analyse results of the average number of regular medications and average number of regular tablets/capsules per patient on each ward.
Counterbalance measures were selected to measure whether the intervention risked causing a negative impact on symptom control. To do this, the number of rescue (PRN) medications were monitored.
X-bar and S charts12 were produced to ensure that the reduction of regular tablet/capsule medications had not been achieved at the expense of a counter increase in liquid medications.
A U chart for the average number of rescue medication required per patient was produced. Initially controlled drugs which were mainly used for pain or breathlessness were included. From 19/09/2015 all ‘as required’ medicines used were included as this was felt to be more accurate.
A C chart12 of length of stay data was also studied to check that interventions did not increase the average length of stay (See supplementary - driver doc for BMJ).
supplementary driver doc for BMJ
Design
An initial education session for the doctors on the level of polypharmacy at the hospice was held and from this the idea was generated of trialling ‘as required’ paracetamol . This was instead of regular paracetamol in patients on strong opioids who are unclear if they are getting any additional benefit from this high frequency and high tablet load medication. Regular paracetamol in step 2 and 3 of the WHO analgesic ladder has little evidence to support its use.13 ,14
Development of a laxative guide encouraging the use of compound laxatives where appropriate was introduced to the doctors during a teaching session on constipation. The guide was also made available on the desktop of the ward computers.
Education on improving the doctor's knowledge of available strengths of common medications used in palliative care was carried out for all prescribing doctors and colourful guides were placed in the prescribing areas. An evaluation was carried out several weeks later which showed that knowledge (assessed by an anonymously filled- in quiz) had increased by 17%.
Patients identified with major polypharmacy (defined as more than 10 regular medications or more than 20 regular tablets/capsules) by the pharmacist or the project's doctor (AP) when carrying out the weekly data collection would have a polypharmacy checklist sticker inserted into their multidisciplinary (MDT) case notes with suggestions based on ‘as required’ paracetamol, use of compound laxatives and dose strengths where relevant. Any other suggestions from the pharmacist could also be added. These patients would then be highlighted in the ward handover book for a polypharmacy medication review on the ward round after the weekend.
Sticker and checklist: A polypharmacy review sticker was designed for use on the medicine chart of patients with major polypharmacy. The sticker was placed under the last prescribed item so that it would be seen by the next person to prescribe on, or to review the chart. The polypharmacy review sticker either alone and/or with a checklist in the notes and/or a pharmacist on the ward round was assessed by a factorial design15. The effectiveness of this process was monitored by collecting data on how many patients identified as having major polypharmacy by the weekly point prevalence count had a polypharmacy review sticker on the medicine chart.
A survey of patients' views on their medication was developed and tested using a PDSA12 design. This survey, which was completed by the interviewer (KC), was simple and quick for patients to answer. Patients that the clinical team felt were too unwell or cognitively unable to participate were excluded. An initial PDSA cycle focused on two patients who expressed that they were taking too many tablets. The doctor caring for them was asked to review the number of tablets the patient was taking. This was then repeated with four patients. These patients were not necessarily patients with major polypharmacy by our definition, but were clearly patients who perceived themselves as patients with medication burden.
Strategy
The strategy is shown in the Driver Diagram (see Diagram 1) and summarised in Table 1 (see Table 1). PDSA cycle 1 aimed to raise the awareness of ward staff about the problem of polypharmacy at the hospice. An educational session was developed which presented baseline data in an engaging format including a ‘cakeometer which was decorated with 72 sweets’. This visually demonstrated the number of tablets an individual patient was taking on a daily basis. We saw an initial improvement in polypharmacy after the education but this wasn't maintained when staff members changed. We realised that education although effective in the short term would not be sustainable due to difficulty in staff being able to attend sessions and changes in staff as people leave or join the hospice. Moving forward, ideas and suggestions from staff were collated and developed into further PDSA cycles.
We continued to collect weekly point prevalence data which we hoped would demonstrate which PDSA cycles were effective. One suggestion was to focus on medicines with a high tablet burden, laxatives and regular paracetamol were both highlighted as problematic. We designed a laxative guideline that was displayed on computer screen savers. The guideline was introduced to prescribers again in an education session alongside other information about constipation and laxatives with a strong focus on reducing tablet burden. (PDSA cycle 2) Initially a 30% reduction in average number of tablets was made. Rationalizing laxatives appeared to be a useful strategy to reduce polypharmacy.
PDSA cycle 3 relied on education sessions for prescribers to raise awareness of different dose strengths available. Lessons learnt from previous education sessions demonstrated that success depended on staff availability and was affected by changes in staffing. Therefore although the education was initially successful alternative methods to improve polypharmacy needed to be explored.
A checklist was developed which focused on key areas such as laxatives, paracetamol and optimising dose strengths. (PDSA cycle 4). This was placed, by the pharmacist, in the medical notes of patients who were identified as having polypharmacy. The aim being that it would be acted upon during the consultant ward rounds. However it was sometimes not prominent enough in the chronological order of events of the notes to be discussed. Alongside the checklist a sticker was developed that would be attached to the medicine chart of patients with polypharmacy to direct prescribers to the checklist in the notes. This was much more visible and by enlisting the help of other members of the multidisciplinary team it did not rely on the pharmacist being available to use it.
PDSA cycle 5 utilised factorial design (sometimes called a ‘planned experiment’) to compare the effect of the checklist, the sticker and a pharmacist's presence on the ward round. Although a positive effect from each variable was shown, it was not possible to demonstrate effectively, from this sample, that one was superior to another. Process measures were required to ensure that checklists, stickers and pharmacists were being utilised effectively.
Although the weekly data was showing some interesting results at this point of the project the impact on patients had not been measured. Was polypharmacy a perceived problem rather than an actual problem for patients at the hospice? A series of PDSAs (cycle 6) were conducted to investigate this in the form of patient surveys. We trialled a pilot survey on one patient, learning that they preferred the investigator to help complete it and the questions needed to be simple and unambiguous. Some patients were excluded as they were identified by nursing staff as being too unwell or unable to complete it. However results showed that polypharmacy is a problem for patients and 87% would like to take less tablets. However the 13% who were happy with the number of medications that they were on were prepared to take whatever number of tablets if it meant better symptom control4 (See supplementary – All PDSAs_0).
supplementary supplementary – All PDSAs_0
Results
From the Control Charts an encouraging decrease in the average number of medicines and regular tablets/capsules was observed after prescriber education sessions and trials of ‘as required’ paracetamol for patients on strong opioids (see Chart 1 and 2: 11 July - 8 August). A decrease in the volume of regular liquid medication was also noted (see chart 3: 11 July - 8 August). A changeover of junior doctors on the ward (see Chart 1, 2 and 3: 22 August) coincided with an increase in average number of regular medicines. Subsequent repeated education combined with the development of a laxative guide again saw the average number of medicines and tablets/capsules fall (see Chart 1: 5 September - 19 September). Laxatives had been identified as a group of medicines that contribute to a large medication burden for hospice inpatients. By making the laxative guide highly visible in the ward area (by use of wall charts and wallpaper on computer desktops) it encouraged the appropriate prescribing of compound laxatives which therefore led to a decrease in tablet/capsule burden.
Improving the knowledge of dose strengths did not reduce the number of regular tablets further, despite the education being effective (see Chart 1: 10 October). At this point, it was not felt that further education, specific to dose strengths, would sustainably reduce polypharmacy at the ward level. However the pharmacist will continue to advise on dose strengths for individual patients.
Developing the polypharmacy checklist for the MDT case notes and subsequently the review sticker for the medicine chart highlights the patient with major polypharmacy who will benefit from a medication review. It is known from PDSA cycles performed on a total of 6 patients, that with half of the patients who felt they were taking too many medicines, a 33%, 36% and 46% reduction in number of regular tablets/capsules was achieved (two died before the review and one did not achieve a reduction). Asking the patient whether they are on too many tablets is a good strategy which benefits all patients, not just those with major polypharmacy. The plan is to incorporate this in the medicines reconciliation process conducted by the doctor and pharmacist/ pharmacy technician at admission.
A 2 to the power of 3 factorial design15 was used to study the effect and relationship between the presence of the pharmacist on the ward round, the checklist of recommendations in the case notes and the sticker highlighting polypharmacy and requesting review on the medicine chart.
The presence of a pharmacist on the ward round and the use of the checklist appeared to be independent to each other. You would expect this as the pharmacist would prompt review whether there was a checklist there or not. However it was felt the presence of the checklist and/or polypharmacy review sticker created a ward round opportunity to focus on review of medication. When a pharmacist was present there was an average reduction in number of regular tablets/capsules of 29%, when the pharmacist was not present there was an average reduction of 15%.
The results where a pharmacist was not present were investigated further. Initial results show an average reduction in the number of tablets of 5% when neither a sticker nor checklists were used. A reduction of 13% for checklist alone, 17% for sticker alone and 25% when a combination of sticker and checklist were used together was seen. When these results are plotted on a response plot the lines are parallel which indicates that the effects from using a sticker or checklist do not interact but they both have an important effect on reduction of average number of tablets.
The presence of a pharmacist on the ward round was shown to produce a good effect but the pharmacist is a limited and costly resource and it is not possible for them to be on all ward rounds. Therefore it was decided to implement the use of a polypharmacy review sticker and a checklist for all patients identified as having major polypharmacy. Both showed good effect either alone or in combination.From the Shewhart chart the introduction of the checklist maintained the improvement in the number of regular tablets and capsules and also regular medication even with the subsequent rotation of doctors (see Charts 1, 2 and 3: 7 November - 6 February).
As the numbers of patients tested in this small scale factorial design were low, it is difficult to interpret these findings with confidence and the test would be more relevant when scaled up to 10 patients per category. With this scenario, it may be predicted that the greatest effect would be observed when +pharmacist +sticker and +checklist are applied.
A process measure needs to be undertaken to check that the sticker and/or checklist are being signed by the prescriber as being considered and acted upon where appropriate.
Initial process measurements have indicated that the pharmacist was able to identify and highlight (by using a sticker and/or checklist) patients with major polypharmacy successfully in 80%-100% of patients.
The process by which patients with major polypharmacy are identified will be strengthened by the involvement of the pharmacy technician to highlight these patients during the initial medicines reconciliation process. Additionally the nursing staff identify patients on greater than ten regular medicines as part of the monthly Medicine Safety Thermometer initiative.16 They have adopted the use of the polypharmacy review sticker to highlight these patients to prescribers.
From the Shewhart charts looking at ‘as required’ medication use during the study period (see Chart 4) and also length of stay during the study period (see Chart 5) there does not seem to have been any adverse effects from this quality improvement (QI) work.
Lessons and limitations
Finding suitable times to conduct the education sessions was challenging and has required repeating sessions on different days in order to ‘catch’ all doctors including the problem of educating rotating doctors who input to the on-call rota alone. To begin with, education appeared to deliver the biggest improvement in polypharmacy yet alone was insufficient to maintain these gains. Achieving improvement often requires change in behaviour, particularly in learnt behaviour. In palliative medicine, the WHO analgesic ladder 17, where regular paracetamol is continued when strong opiates are commenced is an example of where, within this project we attempted to influence embedded behaviour. While initially successful, the lack of sustained improvement led to the development of the pharmacist driven intervention, including the checklist and prescription sticker.
The laxative guide needs to be reviewed in relation to the high cost of combination laxatives. This could be a potential prescribing issue when patients are discharged. Ideally, the generation of accredited guidelines, specific to the field of palliative medicine that considers the problem of medication burden for the patient is needed.
Attempts at cost analysis during the study were made and were found to be unfeasible, due to the difficulty in separating out individual patients' medications. Also, the project focused on quality improvement per se, rather than cost and time reduction.
The calculation of the number of tablets/capsules was based on the BNF strengths available rather than the hospice stock formulary where not all strengths of each medication is stocked.
It is recognised that there is commonly a reduction in the number of regular tablets/capsules as a patient deteriorates and enters the last days of life (unpublished observations). Data from these patients can positively skew the results although this would be expected to be a relatively constant affect over time.
Two of the investigators are clinically involved on the wards at the hospice.
In calculating number of regular medicines, the decision was taken not to include nutritional supplements, emollients or nebulised saline. It is however accepted that these have ‘costs’ to supply and administer and contribute to the overall medication burden for the patient.
Sustainability of these gains is to be monitored monthly. There will be difficulties on weeks when the pharmacist is not present however, the pharmacy technician and nursing staff are well placed to adopt a role in the polypharmacy vigilance process so that it's not dependant on a single individual. With the introduction of electronic prescribing it should be possible to reliably identify the patients with major polypharmacy but the advice of the pharmacist will still be required.
In principle, the interventions are adaptable and scalable to any organisation yet it is recognised that we have discussed this in the context of an individual organisation.
Conclusion
Overall the average regular tablet/capsule burden per patient on the pilot ward was reduced by 25%, the number of regular medications was reduced by 16% and the average volume of liquid medication was brought down by 30%. There was no increase in the use of ‘as required’ medication and no increase in length of stay.
Using QI methods has helped show that the problem of polypharmacy and in particular the tablet/capsule and liquid medication burden for the patient can be reduced by relatively simple means. One method is to ask the patient if they feel they are taking too many medicines. Another method is to identify and highlight patients with major polypharmacy by the use of a sticker on the medicine chart. Supporting this is the use of a checklist completed by the pharmacist and added to the MDT case notes. The pharmacist makes suggestions as to which medicines could be reviewed focusing particularly on high frequency and high tablet count medicines.
While there is no direct comparison for the improvements in this project specifically in the palliative care setting the achievements reflect those reported in the wider context of polypharmacy.1
Further work is planned to look at whether this approach will work in other in-patient hospice settings. Whether this approach would work for outpatient, day hospice and hospital palliative care settings needs to be investigated. The generation of accredited guidelines on polypharmacy specific to the field of palliative medicine is recommended.
Acknowledgments
Kathleen Connors Medical Student Manchester University
The patients and staff at St Ann's hospice who contributed to this project
Haelo for their help and suggestions throughout the Improvement Science 4 Academics programme (www.haelo.org.uk).
Footnotes
Declaration of interests Nothing to declare.
Ethical approval This project was an improvement study at the hospice and ethical approval was not required.