Article Text
Abstract
Objective To examine whether or not self-sampled cervical screening for human papillomavirus (HPV) DNA is acceptable and if women prefer self-sampling to clinician-based sampling.
Design Systematic review and meta-analysis.
Data sources Thirty-seven primary studies obtained through a comprehensive search of six electronic bibliographic databases from 1986 to 2014 and other sources. Search keywords included HPV, screening, DNA testing, vaginal testing, self-collected specimen, self-collected sample, self-sampling, self-screening, preferences and acceptability.
Review methods Studies eligible for analysis included those that had participants perform self-sampling, evaluated participant acceptance of or preference for self-sampled vaginal HPV DNA and reported data to calculate an effect size. There were no exclusion criteria for publication status or geographical location. Meta-analytic methods were used to quantitatively synthesise effect sizes across studies.
Results The 37 studies included 18 516 female participants from 24 countries across five continents. Overall, there was a high level of acceptability of self-sampling among the participants. Participants reported preference for self-sampling over clinician sampling due to attractive characteristics such as ease and privacy.
Conclusions The overall acceptability of self-sampled cervical screening, coupled with economic and effective care, provides opportunities for expanding screening services. Importantly, this can provide a creative screening alternative for women who do not participate in traditional cytological screening, and may ultimately reduce health disparities and prevent cervical disease.
- HPV
- SCREENING
- META-ANALYSIS
Statistics from Altmetric.com
Rationale
Persistent infection with high-risk strains of human papillomavirus (HPV) is the major cause for cervical cancer and other anogential cancers.1–5 There are more than 100 HPV viral types, 13 of which are known to be oncogenic.3–5 Most notably, HPV 16 and 18 collectively account for 72% of HPV-related cancers.4 Historically, strategies to limit the long-term oncogenic effects of HPV focused on early detection of cervical cancer via cervical cytology (ie, Pap testing). More recently, HPV DNA testing has emerged as another strategy for cervical cancer prevention.
HPV DNA testing has a high sensitivity for detecting cervical precursor lesions.6 ,7 Randomised controlled trials suggest that HPV DNA testing in combination with cervical cytology may be a more effective approach for the early detection of cervical cancer for women ≥30 years than cytology alone.8 With regard to invasive cervical cancers, HPV DNA testing results in greater prevention than Pap screening;9 it has higher sensitivity for detecting recurrent or residual high-grade cervical cancer and can do so more quickly than cytology.10
Cervical samples for HPV testing are typically collected by a clinician during a pelvic examination.9 ,11 However, HPV DNA also has the potential to be self-collected. Studies have shown a high concordance for cervicovaginal HPV testing between samples collected by patients and those obtained by clinicians.6 ,7 ,12–14 A meta-analysis found HPV DNA testing of self-samples to have similar sensitivity and specificity to clinician-based screening when used in conjunction with PCR tests.15 Today, the WHO endorses HPV DNA testing in women aged ≥30 years using cervical samples collected by either a clinician or individual via self-sampling.16
Although self-sampling is established as an effective strategy for detecting cervical cancer, it is less clear whether women view this as an acceptable screening option.17 The purpose of this systematic review and meta-analysis was to assess women's acceptability of self-sampling for HPV DNA testing and their preference for self-sampling compared with clinician-collected samples for the purpose of cervical cancer screening.
Methods
Systematic review methods were used for the search, study selection and data extraction. Meta-analytic techniques were employed to quantitatively synthesise estimates of acceptability and preference for self-sampling across studies following the PRISMA reporting guidelines.18 The review protocol was developed a priori and is registered with PROSPERO (Registration #CRD42015016708).
Study eligibility
Studies were eligible for inclusion if they met the following criteria: (1) included participants who performed cervicovaginal self-sampling; (2) measured general acceptability or characteristics of acceptability regarding cervicovaginal self-sampling for HPV testing, or preference towards either self-sampling or clinician-based sampling; (3) reported sufficient data to calculate an effect size; and (4) was published after 1986. We chose studies dated later than 1986 based on two criteria: (1) the first study of HPV DNA testing was reported in 198619 and (2) the first known HPV self-testing article occurred in 1993.20 There were no exclusion criteria for publication status (eg, thesis, ePub or print) or geographical location.
Study search, selection and coding procedures
A systematic strategy was employed to search for studies that met the above inclusion criteria. The search, completed in March 2015, involved six electronic databases (Scopus, Web of Science, PubMed, ProQuest Dissertations and Theses, Cochrane Database of Systematic Reviews OpenGrey), reference lists of included studies and prior related reviews. While database searches have become more reliable with improved technology and indexing, not all reports may be included in the databases or identified with relevant indexing terms, particularly for articles published in supplements or before 1991. Cochrane suggests hand searching in relevant journals as a useful adjunct to electronic database searches.21 Thus, we also searched the following sexual health journals by hand, Sexually Transmitted Diseases, Sexually Transmitted Infections, Vaccine, and Sexual Health, to ensure a robust search of the literature. A librarian was consulted to identify an effective, efficient search strategy in each database using MeSH headings and database-specific subject and keyword terms (see online supplementary appendix for search terms). Search terms included HPV, screening, DNA testing, vaginal testing, self-collected specimen, self-collected sample, self-sampling, self-screening, preferences and acceptability. The specific search strategy for each electronic database can be obtained from the authors.
supplementary appendix
Two authors (JF and RG) independently searched all sources and reviewed titles and abstracts to identify studies for full-text screening. The full text of all studies that were questionable for inclusion at this stage was retrieved and independently reviewed for eligibility using a screening instrument. Discrepancies in screening decisions between reviewers were resolved through discussion and consensus; when necessary, a third reviewer (EJN) was consulted. The two reviewers independently coded all studies that passed eligibility screening using a coding instrument for systematic examination and extraction of data. The coding instrument included categories concerning bibliographic information: study context, sample descriptors, research methods and design, and effect size data. The coding instrument was pilot tested using two studies and revisions were made. After finalisation of the coding instrument, the two reviewers independently extracted data from the eligible studies. Coding discrepancies were resolved by discussion and consensus and, when necessary, a third reviewer (EJN) was consulted.
Effect size measures
Our primary outcome of interest was the proportion of women reporting that self-sampling was acceptable. We defined acceptability based on responses to questions that directly asked women about their overall satisfaction with the self-sampling experience after performing a self-sampling procedure. Composite or index scores that combined responses regarding the characteristics of self-sampling (eg, pain, ease, embarrassment) were not considered as measures of general acceptability. We also measured the proportion of women who reported that they would use self-sampling as a primary screening method in the future as a proxy measurement of acceptability, as women who did not favour the self-sampling experience would likely not be willing to participate in future self-sampling. We also measured characteristics of self-sampling that women reported having liked or disliked. Specifically, we measured the proportion of women reporting ease of completing the procedure, convenience, being able to sample on their own, privacy, embarrassment, comfort performing self-sampling, pain or physical discomfort, anxiety, uncertainty about self-sampling correctly (ie, obtaining sufficient material for testing) and having to touch themselves. As a secondary outcome of interest, we measured the proportion of women who reported preference for self-sampling and/or clinician-based sampling. Preference was determined using responses to questions that asked women directly if they preferred self-sampling, clinician-based sampling or a combination of both methods.
Statistical procedures
We estimated study level effect sizes for all studies that reported sufficient data to do so. Specifically, we estimated the proportion (p) and its accompanying 95% CI as the primary study effect of interest. Using proportions in meta-analyses provides a suitable estimate of the mean proportion across studies, but this method can underestimate the size of the CI and overestimate the degree of heterogeneity, especially as p approaches 0 or 1, due to compression of the SE.22 To account for extreme estimates of acceptability, characteristics of acceptability and sampling preference, we employed the logit method to convert observed proportions to logits for analysis.22 A random-effects meta-analysis was used to estimate the overall proportion of positive responses for acceptability, each of the aforementioned characteristics of acceptability and sampling method preference. Study estimates were combined on the logit scale, and the combined estimate was transformed back to the proportion scale for ease in interpretation. There was one instance where a proportion was equal to 1; therefore, we employed the continuity correction suggested by Nyaga et al,23 where 0.5 was added to the cell counts to retain the study in the analysis. To assess heterogeneity, I2 was computed within each combination. All analyses were performed using the meta (G. Schwarzer. meta: General Package for Meta-Analysis. R package V.4.3-2, 2015.) package in R (R: A language and environment for statistical computing (program). Vienna, Austria: R Foundation for Statistical Computing, 2014.).
Results
Figure 1 summarises the flow of studies through the search and selection process. We retrieved 416 records via electronic databases, with additional citations reviewed from references listed in prior reviews, studies and website searches. After review of titles and abstracts by two independent reviewers and the removal of duplicates, we identified 135 unique reports. Studies were then screened and excluded if they did not measure acceptability or sampling preference or if study participants did not participate in self-sampling (n=72). This resulted in 63 articles that were considered for full-text screening. After full-text screening, a total of 37 studies met eligibility criteria (see supplementary tables S1 and S2 for a complete listing of included and excluded studies) and were included in the quantitative synthesis. In all, 26 studies were excluded because participants did not perform self-sampling (n=15); the study did not measure acceptability, characteristics of acceptability or sampling preference (n=10); or the study did not provide sufficient data to calculate an effect size (n=1).
Flow chart of study search and selection process.
supplementary table
Characteristics of Studies that were Excluded after Full Text Review.
Characteristics of included studies
The 37 studies eligible for analysis included 18 516 female participants from 24 countries across North America, South America, Europe, Africa and Asia. The majority of these studies (n=31) used a cross-sectional survey research design, although four cohort and two experimental studies were also identified. Most were published in peer-reviewed journals (92%), with the remainder being dissertations/theses. All 37 studies involved vaginal or cervicovaginal sampling. Several types of sampling devices were used including swab (n=15), brush (n=9), lavage (n=6), tampon (n=2) and Kato's self-scraping device (n=1); four studies did not provide enough information to identify the sampling device, and others used more than one method. Participants collected the samples in their home (n=9) or in a clinical setting such as a clinic, physician's office or hospital (n=27); one study did not report where self-sampling occurred. Funding sources included governmental entities (n=17), private foundations (n=5), university grants (n=7) or pharmaceutical companies (n=3), with no external funding reported for five studies.
Acceptability of self-sampling
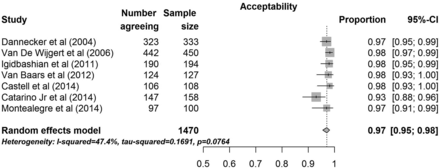
For the primary outcome of general acceptability of self-sampling, there were a total of seven studies which included 1470 women. A random-effects analysis estimated that, on average, 97% of women (95% CI 95% to 98%) found self-sampling to be generally acceptable. Heterogeneity was moderate (I2=47.4%), though all study-specific point estimates were greater than 93%. A forest plot of the studies is given in figure 2. Using the proxy measurement of acceptability (ie, the likelihood of women participating in self-sampling in the future), nine studies with a total of 2660 women were analysed. A random-effects analysis estimated that, on average, 87% of women (95% CI 73% to 95%) would be willing to self-sample again in the future. However, there was large heterogeneity (I2=98.2%) indicating a wide range of estimates for this proxy measurement.
Forest plot showing the proportion of women that considers self-sampling of vaginal material for human papillomavirus testing as generally acceptable. References available in Supplementary Table S1.
Sampling preference
Twenty-three studies asked a total of 12 610 participants whether they preferred self-sampling or clinician-based sampling for HPV testing. The pooled estimate of women reporting preference for self-sampling was 59% (95% CI 48% to 69%). Individual studies ranged from 22% to 95% of respondents reporting preference for self-sampling, with a corresponding I2 of 99%. A forest plot of these results is shown in figure 3. Due to the heterogeneity across studies, studies were stratified based on the response options for questions regarding sampling preference. Studies where participants were given a binary response option of either preference for self-sampling or clinician-based sampling were grouped separate from those in which the participants were given three response options: (1) preference for self-sampling, (2) preference for clinician-based sampling or (3) preference for a combination of self-based and clinician-based sampling. A larger proportion (65%, 95% CI 54% to 74%) of participants reported preference for self-sampling when given the binary choice compared with 31% (95% CI 21% to 43%) of women reporting preference for self-sampling when permitted to choose between self-sampling, clinician-based sampling or both.
Forest plot showing the proportion of women preferring self-sampling as a method to collect material for human papillomavirus testing.
Reasons for preferring or disliking self-sampling
Thirty-four studies examined specific indicators of participants' reasons for preferring or disliking self-sampling. Table 1 summarises the results of the meta-analyses by reason for preference or dislike of self-sampling. The most common reasons reported for preferring self-sampling were ease of use (91%), not embarrassing (91%), privacy (88%), comfort performing self-sampling (88%), ability to sample on their own (69%) and convenience (65%). The most frequently reported reasons for not liking self-sampling were uncertainty about self-sampling correctly (21%), painful or physically uncomfortable (10%), caused anxiety (15%) and not wanting to touch themselves (6%).
Reasons for preference or dislike of self-sampling
Discussion
This systematic review and meta-analysis found that women are overwhelmingly accepting of self-sampling for HPV DNA testing. This sense of acceptability is supported by perceptions about ease and convenience of self-sampling, self-involvement in medical care, privacy and lack of embarrassment. Among those who were less accepting, pain, uncertainty as to whether the sample was collected correctly and discomfort touching oneself were cited as concerns. It is noted, however, that pain is likely to occur even during clinician-based sample collection.24 Characterising acceptability of self-sampling is important because the acceptability of the screening procedure is a key characteristic of a good screening test;25 if women are not accepting of self-sampling for HPV testing, then incorporation of this strategy into screening programmes that recommend HPV testing would be limited.
WHO guidelines recommend cervical cancer screening with an HPV DNA test, followed by treatment of precancerous lesions.26 Currently, HPV DNA testing is recommended in Australia, the USA and many European countries.27–29 The US Preventive Services Task Force recommends a combination of Pap and HPV testing for women aged 30–65 years; those with a negative HPV result can wait 5 years until their next screening.29 However, these guidelines focus on HPV testing using samples collected by clinicians.28
HPV DNA screening for cervical cancer is economic, efficient, effective and versatile.41 ,42 However, self-sampling has the potential to further reduce screening costs, as it eliminates the need for an initial clinical encounter in the screening process.30 Self-sampling may also increase access to screening for women who currently do not access cervical cancer prevention services.31 ,32 For example, approximately 25% of women in the USA have not had a Pap test within the last three years.33 Pap uptake declined from 2003 to 2013 and is lower among those who are uninsured and who have not attended college.33 While the Affordable Care Act may facilitate screening access for women who were previously uninsured, it is possible that even those with insurance may still find that factors such as clinic hours, clinic locations and childcare considerations may hinder the ability to access clinician-based cervical cancer screening. Thus, the ability to self-sample at home and at a time convenient to each individual may facilitate increased screening uptake and reduce costs.
Despite the high levels of acceptability for self-sampling shown in this meta-analysis, self-sampling may have limited impact in areas where cervical cancer burden is highest, namely low/middle-income countries. The WHO recognises that because HPV testing requires higher level resources (eg, laboratories, trained lab technicians, national reference library), it may not be a feasible strategy in areas with limited resources.16 Three studies in low-resource settings examined in-home clinician-based sampling as a method of cervical cancer screening.34–36 Acceptability of self-sampling was not evaluated, but findings did indicate promise for at-home screening options as a way to reach women who can benefit most from screening outreach. Of note, many women in this meta-analysis indicated that they would prefer a combination of self-sampling and clinician-based sampling. This approach aligns with recent efforts to engage individuals in participatory medicine, where patients and clinicians work cooperatively to promote individual well-being.37 ,38 Offering at-home self-sampling with a health outreach worker present may help women engage in participatory medicine and overcome perceived barriers to self-sampling. Thus, self-sampling raises the potential for more women to be up to date with screening in accordance with their risk profile.39 ,40
Self-sampling for HPV DNA testing has the potential to overcome practical and perceived barriers that may impede some women from engaging in cervical cancer screening. Addressing common concerns (eg, pain, anxiety and ability to correctly self-sample) may improve the self-sampling experience and increase its acceptability. More qualitative and quantitative data are needed to assess the efficacy of self-sampling kit instructions, as well as the marketing messages used to promote self-sampling.
While this meta-analysis employed rigorous review methods, it is not without limitations. Although we conducted a comprehensive search for eligible studies, we did exclude those published in a language other than English. Because the majority of the studies included were cross-sectional in design, it is not possible to draw causal inference. Similarly, it is not possible to assess whether women who reported acceptability for self-sampling would actually engage in self-sampling under non-study conditions. However, increased participation rates have been observed among women who self-sampled compared with clinician sampling under controlled settings;41 this supports the potential for self-sampling to reach women who do not participate in regular cervical cancer screening. We observed high levels of heterogeneity with regard to acceptability but could not use meta-regression techniques to quantitatively assess the reason for these differences due to a small sample size. Acceptability did not appear to qualitatively vary by race/ethnicity, location or cultural factors. However, the underlying variations in levels of self-sampling acceptability and preference across studies should be explored as more acceptability studies emerge because this may impact the utility of incorporating self-sampling strategies into cervical cancer prevention programmes. Finally, the small number of studies addressing general acceptability limited our ability to conduct meta-regression analyses to adjust for potential differences. As more acceptability studies emerge, meta-regression techniques should be applied to explore potential differences among specific populations.
This meta-analysis provides a systematic synthesis of the acceptability and preference for HPV DNA self-sampling and lends important insights into the feasibility for potential incorporation of this strategy in cervical screening programmes. Findings indicate that self-sampling for HPV DNA testing is an acceptable screening technique. Women like many of the benefits of self-sampling such as ease and convenience of use, privacy and being directly involved in their healthcare. The overall acceptability of this strategy provides opportunities for expanding screening services, further reducing screening costs and creatively accessing women that do not participate in traditional cytological screening to reduce health disparities and prevent cervical disease.
Key messages
Self-sampling for human papillomavirus (HPV) DNA testing is generally acceptable to women and is preferred to clinician-based sampling.
Incorporating self-sampling strategies into cervical cancer screening programmes will reduce costs and may even increase the number of women reached by these programmes.
Addressing practical and perceived barriers of self-sampling may further increase its acceptability and usage among women.
Acknowledgments
The authors would like to thank Dr Donghua Tao from the Saint Louis University Medical Center Library for her assistance with our database search strategy.
References
Footnotes
Handling editor Jackie A Cassell
Twitter Follow Brandy Maynard at @BrandyRMaynard
Contributors EJN conceived and designed the study. EJN, BRM and LDA drafted the manuscript. TL conducted the statistical analyses. BRM, LDA, TL, JF and RG assisted in study design. All authors participated in editing the manuscript and approved the final protocol.
Competing interests LDA owns <$5000 of stock in Merck.
Provenance and peer review Not commissioned; externally peer reviewed.