Article Text
Abstract
Background Sepsis causes substantial morbidity and mortality in hospitalised patients. Although many studies describe the use of protocols in the management of patients with severe sepsis and septic shock, few have addressed emergency department (ED) screening and management for patients initially presenting with uncomplicated sepsis (ie, patients without organ failure or hypotension).
Objective A quality improvement task force at a large, quaternary care referral hospital sought to develop a protocol focusing on early identification of patients with uncomplicated sepsis, in addition to severe sepsis and septic shock.
Intervention The three-tiered intervention consisted of (1) a nurse-driven screening tool and management protocol to identify and initiate early treatment of patients with sepsis, (2) a computer-assisted screening algorithm that generated a ‘Sepsis Alert’ pop-up screen in the electronic medical record for treating clinical healthcare providers and (3) automated suggested sepsis-specific order sets for initial workup and resuscitation, antibiotic selection and goal-directed therapy.
Design A before and after retrospective cohort study was undertaken to determine the intervention's impact on compliance with recommended sepsis management, including serum lactate measured in the ED, 2 L of intravenous fluid administered within 2 h of triage, antibiotics administered within 3 h of triage and blood cultures drawn before antibiotic administration. Mortality rates for patients in the ED with a sepsis-designated ICD-9 code present on admission were also analysed.
Results Overall bundle compliance increased by 154%, from 28% at baseline to 71% in the last quarter of the study (p<0.001). Bundle, antibiotic and intravenous fluid compliance all increased significantly after launch of the sepsis initiative (eg, bundle and intravenous fluid compliance increased by 74% and 54%, respectively; p<0.001). Bundle and antibiotic compliance both showed further significant increases after implementation of suggested order sets (31% and 25% increases, respectively; p<0.001). The mortality rate for patients in the ED admitted with sepsis was 13.3% before implementation and fell to 11.1% after (p=0.230); mortality in the last two quarters of the study was 9.3% (p=0.107).
Conclusions The new protocol demonstrates that early screening interventions can lead to expedited delivery of care to patients with sepsis in the ED and could serve as a model for other facilities. Mortality was not significantly improved by our intervention, which included patients with uncomplicated sepsis.
- Emergency department
- Information technology
- Quality improvement
- Performance measures
- Critical care
Statistics from Altmetric.com
Introduction
Background
Sepsis, severe sepsis and septic shock are significant causes of morbidity and mortality in hospitalised patients.1 Early and appropriate therapy for patients with sepsis has been shown to improve outcomes.2 ,3 Much of the work to improve timeliness of care focuses on bundling elements of sepsis management for patients with severe sepsis or septic shock. These bundles, based on the Surviving Sepsis Campaign (SSC),4 focus on quickly obtaining lactate levels, white blood cell (WBC) counts and blood cultures, early administration of antibiotics and rapid intravenous fluid resuscitation. The use of bundled care, specifically protocolised resuscitation based on measurable haemodynamic end points, was associated with improved outcomes for patients with severe sepsis and septic shock in several observational studies and the original randomised controlled trial by Rivers et al.2 ,5–7 However, the more recent Protocol-Based Care for Early Septic Shock (ProCESS) and Australian Resuscitation in Sepsis Evaluation (ARISE) trials underscored the importance of both early volume resuscitation and antibiotic administration but failed to show a clinical benefit from early protocolised management.8 ,9
While earlier sepsis recognition and management has been shown to reduce the need for the downstream elements of early goal-directed therapy,6 ,10 few protocols targeting improved sepsis screening have been reported. In one study, a protocol incorporating screening by triage nurses led to an improvement in SSC element compliance.11 Another published computer-assisted protocol to improve sepsis recognition led to increased use of lactate levels in this population.12
Local problem
At the University of Washington Medical Center (UWMC) the burden of sepsis is substantial, with more than 750 inpatients with sepsis, severe sepsis or septic shock managed annually. The emergency department (ED) is the single largest portal of entry for these patients, accounting for 40% of all cases. Review of UWMC ED care from May 2011 through October 2012 highlighted wide variations in provider care of patients with sepsis. Based on these findings, a quality improvement (QI) initiative was implemented in the ED to improve care of patients presenting with signs and symptoms concerning for sepsis.
Intended improvement
The framework for the QI initiative was based on John Kotter’s eight-step model for organisational change.13 In the first phase, creating a climate for change, hospital leadership articulated a vision for UWMC to be a leader in reducing in-hospital deaths.14 A guiding coalition was assembled with the relevant knowledge, expertise, credibility and formal authority to drive this change effort. The multidisciplinary workgroup included physician and nurse champions from the ED and critical care services, pharmacists, quality analysts and computer support personnel.
In the second phase of this change model, engaging and enabling the whole organisation, a broad-based communication strategy was used with all frontline providers and staff. The rationale for this effort was conveyed, question-and-answer sessions held and early barriers identified. Feedback from frontline providers helped to inform new workflows while engendering buy-in. Identifying key milestones and adhering to a strict project timeline ensured early successes and short-term wins. The final phase of this model, implementing and sustaining change, was facilitated by the development of a sepsis dashboard to track progress. Results were reviewed regularly by the workgroup, updates given to executive leadership and progress communicated routinely in various quality forums throughout the organisation.
Methods
Setting
Located in Seattle, Washington, UWMC is a 450-bed academic hospital managing more than 18 000 inpatient admissions each year. As a quaternary care facility serving the northwest region, UWMC provides care for one of the most medically complex population of patients in the country. The Medicare Case Mix Index (CMI) of 2.33 for calendar years 2012–2013 ranks in the top 10 of the 240+ academic medical centres listed in the University HealthSystem Consortium.
The multidisciplinary team initially met in August of 2011, at which time the scope of work was defined and project charter developed. The objective of this effort was to develop a process to promote standardisation of care and earlier recognition of patients with sepsis in a manner that aligned with the SSC bundle elements. The ED sepsis bundle compliance report was composed of the following elements:
Percentage of cases with serum lactate measured in the ED
Percentage of cases receiving at least 2 L of intravenous fluid within 2 h of triage
Percentage of cases receiving antibiotics within 3 h of triage
Percentage of cases having blood cultures drawn before antibiotic administration
Constraints placed on the team were that any solution needed to work without hiring additional staff, or significantly increasing capital or operational expenditure. To understand the current state of sepsis care in the ED, team members directly observed and elicited feedback from frontline physicians and nursing staff in order to create a workflow map (see figure 1). Based on this analysis, a multifaceted protocol was created, focusing on early screening, computer-assisted case identification and the use of standardised order sets for evaluation, treatment and resuscitation. The design and implementation phase, from November 2011 to February 2012, focused on identification of necessary resources, development of protocols and construction of order sets.
Preintervention ED sepsis flow map analysis. CBC, complete blood count; ED, emergency department; ICU, intensive care unit; MD, medical doctor/provider; RN, registered nurse.
Intervention
Changes to the ED triage process, use of electronic alerts and computer-based decision support services were the basis for improving care of patients with sepsis presenting to the ED. Triage nurse responsibilities include collecting and documenting vital signs in the electronic medical record (EMR), assessing severity of illness and screening for potentially life-threatening conditions such as acute stroke and myocardial infarction. Given the time sensitivity in recognising sepsis and the impact of escalating care on reducing mortality, screening for sepsis was also introduced at triage. The revised triage form highlighted the criteria for systemic inflammatory response syndrome (SIRS) and prompted the nurse to determine if there was a concern for infection. This determination was based on clinical judgement of the nurse and/or presence of specific clinical findings (eg, altered mental status, indwelling catheter or known immunosuppressed state). Any patient who met two or more SIRS criteria and screened positive for concern for infection was flagged as possibly having sepsis and care was expedited through a nurse-initiated sepsis protocol. Stat laboratory tests including complete blood count, basic metabolic panel, urinalysis and lactate were sent. Blood cultures were obtained. After securing large-bore intravenous access, the first litre of crystalloid resuscitation was infused (unless contraindicated), prior to the ED physician being notified and seeing the patient.
After triage, initial patient data (room location, age, chief complaint) populate a patient tracking board, known as the ‘whiteboard’, visible to all healthcare personnel in the ED. With the initial roll out of the protocol, if a patient was identified as having possible sepsis, a sepsis icon was manually activated to appear next to that patient's name on the whiteboard. The sepsis icon was developed in response to provider and staff feedback that a visual cue would elevate awareness among team members and help prioritise initial workup and management of these patients. In February 2012, the sepsis icon alert was automated by developing a computer-based algorithm in the EMR to continuously screen for all SIRS criteria. An advantage of the automated surveillance system was the ability to include vital signs (ie, temperature, heart rate and respiratory rate) and also WBC count in determining SIRS criteria. In the initial iteration, only triage vital signs were used for this determination. As a result of this change, sensitivity increased for the recognition of patients who only met SIRS criteria after the results of their WBC count returned.
An additional improvement was the creation of the ‘Sepsis Alert’ pop-up screen in the EMR. If the surveillance system identified that a patient met SIRS criteria and the concern for infection field was not initially marked at triage, any provider entering the EMR would receive a ‘Sepsis Alert’ pop-up screen stating the presence of SIRS criteria and querying whether there was concern for infection (a check box). If the provider responded ‘YES’, the whiteboard icon was automatically activated. Subsequent to case identification, the ‘Sepsis Alert’ pop-up screen outlined key interventions and prompted treatment.
Order sets were developed to direct clinical care for patients identified as high risk for sepsis based on initial screening. In addition to the nurse-initiated sepsis bundle, a provider-driven order set was also developed. The goals of order sets were to standardise (1) initial workup and resuscitation, (2) source-specific antibiotic selection and (3) institutionally modified early goal-directed therapy. In an effort to expedite care delivery and reduce variability, triggering of the sepsis alert automatically pulled up the sepsis order set as a potential option for the clinician. Ultimately, the provider could choose to accept, reject or ignore the proposed pathway.
The revised workflow, reflecting the process improvement efforts addressing sepsis care in the ED, is depicted in figure 2. To prepare staff for project implementation, a sequential, multifaceted education and awareness programme for all members of the ED care team was enacted prior to project ‘Go-Live’. Elements of the sepsis screening and bundle elements were communicated broadly and displayed on the ED Sepsis Badge worn by providers and staff (see figure 3).
Postintervention ED sepsis flow map analysis. CBC, complete blood count; ED, emergency department; EMR, electronic medical record; HCW, healthcare worker; ICU, intensive care unit; MD, medical doctor/provider; RN, registered nurse; SIRS+, patient meets SIRS criteria; SIRS−, patient does not yet meet SIRS criteria; WBC, white blood cell.
ED sepsis badge. ED, emergency department; SIRS, systemic inflammatory response syndrome; WBC, white blood cell.
Evaluation
Initially, in order to determine the burden of sepsis at UWMC and better understand baseline performance for the care of these patients, a retrospective analysis was performed from July 2011 to June 2012. Cases of sepsis were identified based on ICD-9 hospital discharge codes for sepsis (995.91), severe sepsis (995.92), septic shock (785.52) as well as pathogen-specific sepsis code (038. series). A manual chart audit was performed to validate accuracy of the coded data. Patients coded with sepsis present on admission (POA) with ED as portal were included in the reference population.
To determine the impact of the new care pathway on SSC bundle elements and provide feedback to frontline providers, a robust monitoring system was developed. A report was created in 3M Health Data Management System to identify all patients with a sepsis-designated ICD-9 code POA who presented to the ED. At UWMC, inpatient registration and care documentation occurs in multiple source systems which flow into a repository Structured Query Language Server database (Amalga) from which data can be retrieved and analysed. The 3M report was cross-referenced with the Amalga database to obtain all relevant information pertaining to clinical care delivery, including time to antibiotics, volume and timing of intravenous fluids and lactate measurements. Manual chart review was performed to validate accuracy of data and abstract any missing information. Information on all patients with sepsis presenting via the ED was ultimately merged into an access database and updated on a monthly basis.
A list of outlier cases (ie, one or more bundle elements were not met) was then sent to the ED physician champion for further review. Cases were adjudicated based on criteria established by the multidisciplinary group. Exclusion criteria for all bundle elements were the following: reasonable diagnostic uncertainty, triage vitals/initial WBC did not meet SIRS criteria, goals of care not consistent with aggressive resuscitation and difficult intravenous access. Additional exclusion criteria for the intravenous fluid bolus metric included decompensated heart failure, end-stage renal disease, haemodialysis dependence, ventricular assist device support and extreme age (greater than 80-years-old) without haemodynamic compromise.
Any case that involved one or more exclusion criteria was omitted from the analysis. The working group met bi-weekly initially (and later monthly) to review progress with SSC bundle compliance (as reported in a monthly dashboard), discuss ongoing barriers and identify solutions. The ED physician and nurse champion provided general and case-specific feedback to providers and staff as well as updates regarding overall performance and trends.
Pearson's χ2 analyses were applied to the compliance and mortality data. Compliance metrics were categorised as (1) baseline, (2) after Go-Live but prior to automated alerts and (3) after automated suggested order sets. These assignments allow comparison of the baseline phase with the study phase before and after automated decision support tools. Data analysis was conducted with SPSS V.22.0 (SPSS, Chicago, Illinois, USA).
Results
Baseline performance
During the baseline period of July 2010 to June 2011, the majority of patients with sepsis (57%) presenting via the ED were admitted to the medical–surgical floor, a trend that remained stable throughout the study period. Review of UWMC ED sepsis care from May 2011 through October 2011 identified deficiencies in sepsis care delivery. Under-resuscitation with intravenous fluids was found in 54% of cases, delays in antibiotic administration in 54% of cases and no lactate screening in 27% of cases.
Timeline of protocol implementation
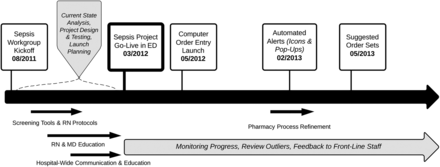
Initial protocol elements were introduced in March 2012 and included the nurse triage screening tool, the nurse-initiated sepsis order set and provider order sets for initial resuscitation, antimicrobial therapy and early goal-directed therapy. Computer order entry was introduced in the ED in May 2012. Automated sepsis surveillance and alert systems for both the white board and EMR were introduced in February 2013. The provider order sets for standardising sepsis care were introduced in May 2013. A chronology of the ED sepsis programme is summarised in figure 4.
ED sepsis project timeline. ED, emergency department; MD, medical doctor/provider; RN, registered nurse.
Bundle compliance
A total of 1032 cases admitted via the ED with sepsis POA were identified in the reported postintervention period. Of these, 624 cases were ultimately included in the study analysis and 408 cases (40%) were excluded from data inclusion. Of those excluded cases, 124 (30%) did not have sepsis identifiable on ED presentation and initial laboratory results. Reasonable diagnostic uncertainty was the other dominant reason for exclusion, accounting for 27% of intravenous fluid outliers and 72% of antibiotic administration outliers.
During the baseline period, overall bundle compliance was 28%. Launch of the sepsis initiative resulted in a 154% increase in overall bundle compliance (see figure 5), peaking at 71% in the most recently reported quarter. Improvements were evident in patients admitted to the floor as well as to the intensive care unit (ICU). Institution of nurse triage screening tool, nurse-initiated sepsis order set and provider order sets increased total SSC bundle compliance to 50%. Introduction of the automated sepsis icon and EMR alerts resulted in further performance improvement to 70% compliance. The implementation of computer order entry and suggested order sets had less discernable effects on bundle performance. There was sustained improvement in all specific bundle elements. Baseline acquisition of blood cultures prior to antibiotic delivery was 90% and never less than 96% after launch of the sepsis initiative. Antibiotic delivery within 3 h increased from 46% at baseline to 82%. Two litre fluid bolus within 2 h improved from 46% at baseline to a peak of 81%, while initial lactate measurement rose from 63% at baseline to a peak of 97%.
Emergency department (ED) sepsis metric compliance performance. Data collection began with Go-Live on 5 March 2012. Baseline data=May 2011 to October 2011 discharges. Calculations based on discharge date, reported in fiscal quarter (Q).
Bundle, antibiotic and intravenous fluid compliance all increased significantly after Go-Live compared with baseline (74%, 30%, 54% increase; p<0.001, p=0.008, p<0.001 respectively), and after suggested order sets compared with baseline (129%, 63%, 66%; p<0.001, p<0.001, p<0.001). Bundle and antibiotic compliance both increased significantly after suggested order sets when compared with after Go-Live (31%, 25%; p<0.001, p<0.001), while intravenous fluid compliance did not change significantly (8%; p=0.163).
Clinical outcomes
While a steady improvement in performance metrics was noted over time, ultimately the objective of the sepsis initiative is to improve patient outcomes. The mortality rate for patients admitted with sepsis presenting via the ED before project implementation (January 2011 through February 2012) was 13.3%, while the mortality rate after implementation (March 2012 through June 2014) fell to 11.1% (p=0.230). The mortality rate in the calendar year prior to implementation was 14% versus 10.4% in the calendar year after (p=0.116). The improvement has been sustained as mortality rate for septic patients presenting via the ED has remained at 9.3% through the first two quarters of 2014 (p=0.107).
Discussion
This paper reports one academic medical centre's experience with the development and implementation of an ED sepsis care pathway, applicable to all patients presenting with concern for sepsis. This unique initiative focused on early recognition through nurse triage, expedited care through shared management between the nurse and physician, dissemination of positive sepsis screen through information technology innovations and reduction in unnecessary variability through the development of standardised order sets.
Mortality rates were used to determine the clinical impact of the process improvement effort. As CMI has remained stable from the baseline and throughout the intervention period, a reduction in the overall sepsis-related mortality rate best reflects improvement in care delivery. While steady improvement in bundle compliance correlated with a reduction in sepsis-related mortality, this trend was not found to be statistically significant. As the duration of our baseline adjudicated data is limited, mortality is reported for all patients with sepsis in the ED. Exclusion of adjudicated cases might alter mortality significance, especially in light of the number of patients determined not to have sepsis identifiable early in the ED. Inclusion of patients with uncomplicated sepsis also confounds interpretation, possibly dampening the effects on mortality. Given the modest baseline mortality patients with sepsis at our centre, demonstrating robust outcome effects proved difficult. Other concurrent QI efforts in the ED and the ICU may have also impacted clinical outcomes.
The protocol initially focused on screening at the earliest possible encounter in the ED: the initial triage nurse evaluation. The data show a rapid improvement in bundle compliance with introduction of triage nurse-initiated screening and order sets, consistent with the findings of another recently reported study.11
A deficiency in the initial screening process was that it was based only on the vital signs present at triage. Baseline analysis of the septic population revealed that 30% of patients with sepsis presenting to the ED met SIRS criteria based on an abnormal WBC count; however, these data were not available at triage. As a result, there was risk that a patient with sepsis would not be identified at the time of triage, resulting in delays in care. Furthermore, many patients had subtle abnormalities in vital signs that met SIRS criteria but were not reliably recognised by providers as abnormal and thus did not consistently prompt consideration of sepsis. To overcome these barriers, a technology-based solution was pursued. Electronic alerts and computer-based decision support systems (CDSSs) have been used effectively in many aspects of patient care including preventive care and appropriate antibiotic prescription.15–17 Given that a computer-assisted sepsis screening process has been shown to be effective,12 ,18 a similar mechanism was pursued. Specifically, a constant surveillance system, including WBC results in SIRS screening, could improve sensitivity while an automated alert could raise awareness of providers and staff of the possibility of sepsis in a patient with more subtle findings on presentation. Indeed, the introduction of this CDSS resulted in a dramatic improvement in total bundle compliance.
To improve consistency of early treatment, ED sepsis order sets were developed. Order sets are a primary mechanism to accomplish bundle compliance, and in the case of severe sepsis and septic shock, have been shown to improve time to antibiotics18 and, in some studies, length of stay and mortality.19 ,20 However, data are limited on the use of order sets for patients with uncomplicated sepsis. UWMC order sets were designed for both nurses and providers. The triage nurse-driven orders were implemented at the beginning of the study period and, in conjunction with screening itself, led to an improved SSC bundle compliance. In addition, a major strength of the standardised order set for physicians was that it guided empiric antibiotic selection based on the constellation of symptoms and signs on presentation and was informed by institutional-specific antibiotic nomograms. As a result, unnecessary variation in ordering practices could be reduced while promoting appropriate coverage for the suspected pathogens.
During the course of project implementation and maintenance phase, results of the much-heralded ProCESS trial were released which cast some doubt as to the utility of standardised care for patients with sepsis in the ED. This prospective multicentre randomised, controlled trial enrolled almost 1400 patients presenting to the ED with septic shock to one of three intervention arms. Findings revealed that protocol-based resuscitation in the ED was not superior to usual care in improving outcomes. It is important to recognise that even prior to randomisation, patients in all three treatment arms received on average at least 2 L of intravenous fluid and 75% were treated with empiric antibiotics within 3 h prior to randomisation.8 Similarly, the ARISE trial failed to show a clinical benefit from early goal-directed therapy, but patients in both cohorts received, on average, 2.5 L of intravenous fluid prior to randomisation and antibiotics within 1.5 h of presentation.9 It is likely that in both trials early recognition with expedited fluid resuscitation and antibiotic administration were key factors in the favourable mortality outcomes, thus validating the use of ED sepsis care pathways focusing on these goals.
There are several potential limitations to this QI-driven intervention. Despite validating the accuracy of data through manual chart review, baseline and study cases were identified using ICD-9 codes which are imperfect tools and may miss some cases of sepsis. The study was a retrospective, before-after design which lends itself to chronology bias and positive results could be the effect of a general improvement in overall ED care or from a concurrent sepsis education initiative. The case exclusion process involves the subjective interpretation of a retrospective chart review. The studied protocol was only implemented in one centre and, as with most QI projects, the protocol would likely need to be modified to be useful in other settings. As both nurse-driven screening and nurse-driven protocols were implemented at the same time, it is unclear which intervention has led to the initial improvement in achieving process benchmarks. It is similarly difficult to establish the exact contribution of other aspects of the protocol to the results.
One concern with more aggressive screening in the setting of computer assistance is provider alert fatigue. This is a well-known limitation of CDSSs, and often results in clinicians overriding or ignoring alerts.21 Alert fatigue and clinician overrides tend to increase as the specificity of the alert decreases.21 ,22 An attempt was made to reduce alert fatigue by limiting the pop-up screen to only the initial time opening of the chart by a given provider.
Understanding the continued barriers to maximising bundle compliance is complex. On investigation of the last reported quarter, 29% of cases did not meet bundle compliance; 14% of these cases failed bundle compliance by only one element (6% for intravenous fluids and 8% for antibiotics). Further, the vast majority of cases received intravenous fluids (97%) and antibiotics (99%) in the ED prior to admission, though not within the stated metric goals (20% received insufficient and/or delayed intravenous fluids and 17% of cases received antibiotics after 3 h). Despite aggressive and ongoing awareness efforts there remains variation in provider engagement, especially with respect to patients with uncomplicated sepsis for whom there is a limited literature to support or refute aggressive treatment. The effects of the fluctuations in ED patient volume and crowding are difficult to quantify but undoubtedly affect the speed at which care is delivered to relatively stable patients. The process from order to administration of antibiotics has many steps allowing for the introduction of systemic delays. In the academic setting, with a constantly changing workforce of rotating learners, it is difficult to get consistent response to and use of the various decision aids. We are continuing to investigate the cultural and systemic factors contributing to the continued gaps in our care delivery. In addition to continuing to elevate awareness and understanding of the protocol and our performance, we are reanalysing our entire process to ascertain where refinements are warranted.
Conclusion
This experience demonstrates how early screening interventions can lead to expedited delivery of care to patients with sepsis in the ED. Other hospitals can incorporate many of the same principals into local QI projects. This protocol emphasises (1) inclusion of all patients with sepsis, (2) screening and enacting workups at the points of initial nursing and provider contact and (3) automated decision support with computer signalling mechanisms. This approach is novel, underused and, as the results show, effective at delivering early care to patients, and thus worthy of consideration at other institutions.
Acknowledgments
Ursula Peavy, RN, BSN; Timothy N. Huff, MHA; Jane Cardoso, MSc, CPHQ; Melissa Irwin, RN, BSN.
References
Footnotes
Twitter Follow Matthew Wemple at @wemplem
Contributors All listed authors easily satisfy the four ICMJE recommendations for authorship. In addition (but not limited to), MOG contributed to the creation of figures, data analysis and statistical analysis, drafting of the manuscript, has been/continues to be the Emergency Department Lead/Champion since the QI project inception, has been intricately involved in all phases of the project planning/implementation/maintenance, and has been the case adjudicator in the sepsis process described in the submission. MW contributed to the creation of figures, drafting of the manuscript, literature review and reference management and participated in the planning and implementation of the sepsis project with heavy input into standardised order sets and modified early goal-directed treatment. PAK has been/continues to be the Critical Care Lead since the QI project inception and has been intricately involved in all phases of the project planning/implementation/maintenance as well as manuscript planning and editing. RD has been/continues to be the Chair of the Sepsis QI Project described since inception, has been intricately involved in all phases of the project planning/implementation/maintenance, as well manuscript planning and editing, and also contributed to the data acquisition, data analysis and statistical analysis.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data not included in this work but also acquired during the ongoing QI project are available to University of Washington employees on the institution's Quality and Safety Dashboard.