Abstract
Background: Drug use increases with advancing age, and in older patients it is associated with an increase in adverse drug reactions (ADRs). ADRs are a primary cause of morbidity and mortality worldwide.
Objectives: To evaluate the prevalence, clinical characteristics and avoidability of ADR-related hospital admissions in elderly patients.
Methods: From November 2004 to December 2005, all patients aged ≥65 years consecutively admitted to the Geriatric Unit of the Casa Sollievo della Sofferenza Hospital, San Giovanni Rotondo in Italy, were evaluated for enrolment in the study. ADRs were defined according to the WHO Adverse Reaction Terminology system. Drugs were classified according to Anatomical Therapeutic Chemical classification system. The Naranjo algorithm was used to evaluate the relationship between drug use and the ADR (definite, probable, possible or doubtful) and Hallas criteria were used to evaluate the avoidability of the ADR (definitely avoidable, possibly avoidable or unavoidable). All cases of a suspected ADR were discussed by a team trained in drug safety, including three geriatricians, one clinical pharmacologist and one pharmacist. Only cases of an ADR with an agreement ≥80% were included.
Results: Of the 1756 patients observed, 102 (5.8%, 42 males, 60 females, mean age 76.5 ± 7.4 years, range 65–93 years) showed certain (6.8%) or probable (91.2%) ADR-related hospitalization. Gastrointestinal disorders (48 patients, 47.1%); platelet, bleeding and clotting disorders (20 patients, 19.6%); and cardiovascular disorders (13 patients, 12.7%) were the most frequent ADRs. NSAIDs (23.5%), oral anticoagulants (20.6%), low-dose aspirin (acetylsalicylic acid) [13.7%] and digoxin (12.7%) were the drugs most frequently involved in ADRs. Of the ADRs, 45.1% were defined as definitely avoidable, 31.4% as possibly avoidable, 18.6% as unavoidable and 4.9% as unclassifiable. Of 78 patients with definitely or possibly avoidable ADRs, 17 patients (21.8%) had received an inappropriate prescription, 29 patients (37.2%) had not received a prescription for an effective gastroprotective drug concomitantly with NSAID or low-dose aspirin treatment and 32 patients (41%) were not monitored during drug treatment.
Conclusion: In the elderly, almost 6% of hospitalizations are ADR related. Most of these ADRs are potentially avoidable. Strategies that reduce inappropriate prescriptions and monitoring errors, as well as improving active prevention of ADRs, are needed in elderly subjects.
Similar content being viewed by others
Background
It is well known that the prevalence of drug use increases with advancing age. Studies have demonstrated that elderly outpatients[1] and hospitalized patients[2] take many medications and that this high rate of drug consumption is associated in older patients with an increase in the number of adverse drug reactions (ADRs).[3] Retrospective studies have demonstrated that ADRs increase the risk of hospital admissions[4,5] and are important causes of morbidity and mortality.[6,7] A prospective, multicentred study carried out on 18 820 patients admitted to two hospitals in the UK reported that ADRs imposed a heavy financial burden on the National Health Service, mainly as a result of high morbidity and mortality rates.[8] Other data collected from populations living at home indicate that between 13% and 27.6% of ADRs are avoidable,[9,10] whereas in nursing homes up to 51% of all ADRs may be preventable.[11] It has been reported that inappropriate drug prescriptions and medication errors may be responsible or 3–9% of hospital admissions and that preventable ADRs may occur in up to 4% of patients during their hospital stay.[12] Because ADRs are a clinically important problem in geriatric patients, prospective studies on their prevalence, clinical characteristics and avoidability carried out specifically in elderly hospitalized patients would be useful in developing preventive strategies.
The aim of this study was to evaluate in an elderly population of southern Italy: (i) the prevalence of ADR-related hospital admissions; (ii) the drugs involved; (iii) the clinical characteristics; and (iv) the potential avoidability of the ADR.
Methods
Study Population
The study was conducted according to the Declaration of Helsinki and the guidelines for Good Clinical Practice, and was approved by local ethics committees (N.3877/DS/2004). Written informed consent was obtained from the patients or relatives of critically ill patients prior to participation in the study.
All patients aged ≥65 years who were consecutively admitted to the Geriatric Unit of Casa Sol-lievo ella Sofferenza Hospital in San Giovanni Rotondo in Italy from 1 November 2004 to 31 December 2005 were evaluated to determine whether hospital admission could be related to an ADR. In all patients, demographic and clinical data were collected by a structured interview and clinical visits including standard laboratory and instrumental tests. In all patients, a Comprehensive Geriatric Assessment CGA)[13] was performed using activities of daily living (ADL), instrumental activities of daily living (IADL), the Short Portable Mental Status Questionnaire (SPMSQ), the Cumulative Illness Rating Scale (CIRS), the Mini Nutritional Assessment (MNA) and social aspects. Moreover, marital status, cohabitation status and education level were recorded.
Drug Use
Medication use was defined according to the Anatomical Therapeutic Chemical (ATC) classification system.[14] The number and doses of drugs taken by the patients were collected by a structured interview with patients and/or their relatives and/or their caregivers at admission. When the drug history was unclear, further data were collected from the patients’ general practitioners’ medical records.
Patients were defined as drug users if they took medication from any of the drug classes included in the ATC classification at the time of admission.
Definition of Adverse Drug Reaction (ADR)
The definition of an ADR used was that of Edwards and Aronson,[15] i.e. “an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product”. Severe ADRs were defined as ADRs associated with life-threatening and/or prolonged hospi-talization and the cause of hospitalization or determining factors for permanent or significant sequelae.[15]
Patients were categorized as having an ADR if the cause of admission was defined according to the WHO Adverse Reaction Terminology (WHO-ART) system,[16] i.e. if there was a temporal relationship between the start of drug therapy and symptomatology, and if, after appropriate investigations, other causes that could explain the symptoms were excluded. This definition excludes therapeutic failures, intentional and accidental poisoning (i.e. overdose) and drug abuse. Accordingly, patients with a suspected ADR related to deliberate non-compliance or intentional/unintentional overdose of drugs were excluded from the study. At admission to the geriatric unit, all patients were initially evaluated by the ward geriatricians. All patients categorized as having a suspected ADR were further assessed by a team trained in drug safety that included at least three of the four geriatricians on the team (Marilisa Franceschi, Carlo Scarcelli, Valeria Niro, Alberto Pilotto), ne clinical pharmacologist (Giovanni Pepe) and one pharmacist (Anna Maria Colusso); only cases with a consensus agreement ≥80% were considered to be ADR cases and were included in the study. In order to assess possible states of digitalis poisoning or bleeding, we considered clinical signs and symptoms, as well as laboratory results and instrumental analyses. When a patient was categorized as having an ADR, this was officially filed with the Italian Ministry of Health. Drug-drug interactions were evaluated according to the Thomson MICROMEDEX Healthcare Series.[17]
Assessment of Causality of the ADR
Assessment of causality of the ADR was performed in all cases using the scoring system derived from the Naranjo algorithm.[18] ADRs were classified as ‘definite’ (score from 9 to 12), ‘probable’ (score from 5 to 8), ‘possible’ (score from 1 to 4) or ‘doubtful’ (score from 0 to –2).[18] A ‘definite’ reaction was one that: (i) followed a reasonable temporal sequence after drug consumption or one in which a toxic drug level had been established in body fluids or tissues; (ii) followed a recognized response to the suspected drug; and (iii) was confirmed by improvement on withdrawal of the drug and reappearance upon re-exposure. A ‘probable’ reaction was defined when (i) it followed a reasonable temporal sequence after drug assumption; (ii) it followed a recognized response to the suspected drug; (iii) it was confirmed by withdrawal but not by exposure to the drug; and (iv) it could not be reasonably explained by the known characteristics of the patient’s clinical state. A ‘possible’ reaction was defined when (i) it followed a reasonable temporal sequence after drug assumption; (ii) it followed a pattern possibly definable as that related to the suspected drug; and (iii) it could be explained by characteristics of the patient’s disease. A reaction was defined as ‘doubtful’ if it was likely to be related to factors other than drug consumption.
Assessment of Avoidability of the ADR
Avoidability of the ADR was assessed using the following definitions developed by Hallas et al.[19] and integrated by Gurwitz et al.,[10] as follows.
-
1
‘Definitely avoidable’ : the ADR was the result of a drug treatment procedure inconsistent with current knowledge of good medical practice, i.e. it satisfied at least one of the following criteria:
-
• the patient had not taken a drug able to reduce or prevent the symptoms according to the prescriptive official procedure;
-
• it was known that the patient was allergic to the drug that had caused the admission;
-
• the patient had a pathology or condition for which the drug was contraindicated;
-
• the patient took a drug that was inappropriately prescribed for the diagnosed disease;
-
• wrong drug/wrong therapeutic choice errors;
-
• wrong dose errors;
-
• prescription of drug associated with a well established clinically important interaction.
-
2
‘Possibly avoidable’ : the prescription was not erroneous but the drug event could have been avoided by an effort exceeding the obligatory demands of current knowledge of good medical practice.
-
3
‘Unavoidable’: the ADR could not have been avoided by any reasonable measures.
-
4
‘Unclassifiable’ : information was contradictory or insufficient to determine the avoidability of the event.
Comprehensive Geriatric Assessment
CGA was carried out and included assessment instruments used widely in geriatric practice. Functional status was evaluated by the ADL index,[20] which defines the level of dependence/independence of six daily personal care activities, including bathing, toileting, feeding, dressing, urine and bowel ontinence and transferring (in and out of bed or chair). The IADL scale was also used,[21] which assesses independence in eight activities that are more cognitively and physically demanding than ADL, including managing finances, taking medications, using the telephone, shopping, using transportation, preparing meals, doing housework and washing. Cognitive status was assessed by the SPMSQ, a ten-item questionnaire that assesses orientation, memory, attention, calculation and language.[22] Comorbidity was examined using the CIRS,[23] which uses five-point ordinal scales (score 1–5) to estimate the severity of pathology in each of 13 systems, including cardiac, vascular, respiratory, eye-earnose-throat, upper and lower gastrointestinal, hepatic, renal, other genitourinary, musculo-skeletal and skin, neurological, endocrine-metabolic and psychiatric. Based on the ratings, the two following scores are derived: (i) the CIRS Comorbidity Index (CIRS-CI) core, which reflects the number of concomitant diseases and is derived from the total number of categories in which moderate or severe levels (grades from 3 to 5) of disease are quoted (range from 0 to 13); and (ii) the CIRS Severity Index (CIRS-SI), which reflects the overall severity of diseases and the average rating of 13 disease categories, excluding psychiatric behavioural problems range from 1 to 5). Nutritional status was explored with the MNA,[24] which includes information on (i) anthropometric measures (body mass index [bodyweight/height2], mid-arm circumference [cm], calf circumference [cm] and weight loss); (ii) lifestyle, medication and mobility; (iii) number of meals, food and fluid intake and autonomy of feeding; and (iv) self-perception of health and nutrition. Social aspects include household composition, home services and institutionalization.
Clinical Laboratory Test Data
Upon admission to the hospital, serum samples were taken from patients treated with warfarin and/ or digoxin therapy to determine the levels of digoxin and haemoglobin, and prothrombin time. The therapeutic range of digoxin was defined at 0.8–2.0 ng/ mL. [10] Prothrombin time was measured and expressed as an international normalized ratio (INR). For patients with mechanical heart valve prostheses, the target intensity (range) was an INR of 3.5 (3.0–4.0). The target intensities (range) for atrial fibrillation and myocardial infarction were INRs of 2.5 (2.0–3.0) and 3.0 (2.5–3.5), respectively. Values above this range were considered toxic.
Statistical Analysis
Statistical analysis was performed with the SPSS (Chicago, IL, USA) software program for Windows (version 13.1). Data were first analysed by descriptive statistics. Quantitative data are shown as mean ± standard deviation. The Mann-Whitney U test was used to compare males with females for the following parameters: mean age, education level, ADL, IADL, SPMSQ, CIRS-CI, MNA, number of drugs and cohabitation status and for their respective proportions, which were analysed by the z test. A pvalue <0.05 was considered statistically significant.
Results
Prevalence of ADRs as a Cause of Hospital Admission
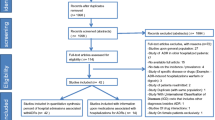
Of the 1756 patients admitted to the geriatric unit during the study period, 105 subjects were identified as suspected ADR cases. Three cases of suspected ADR were excluded from the study because the team considered them to be doubtful cases with an agreement <80%. Thus, the final evaluation included 102 patients with an ADR (5.8% of the total hospital admissions): 42 males and 60 females, with a mean age of 76.5 ± 7.4 years and a range from 65 to 93 years. Seven patients had a definite ADR (6.8%), 93 patients had a probable ADR (91.2%) and two patients (2.0%) had a possible ADR as a cause of admission to the hospital. According to the Italian Ministry of Health, all ADRs that were the cause of hospital admission were defined as severe. In three (2.9%) of 102 patients, the ADR was judged to be life threatening (all three patients had a haemorrhage). At discharge from the hospital, 66 patients (64.7% of the ADRs) had a complete resolution of the ADR, and 36 patients (35.3%) had a clinical improvement.
Functional Characteristics of Patients
Table I shows demographic and functional characteristics of patients included in the study according to sex. Patients with ADRs frequently presented multiple dysfunctions in the ADL (40.4%) or IADL (65.6%), whereas 15.9% of patients had cognitive impairment. More than 53.6% of the patients were at risk of malnutrition or were malnourished and about 27% were living alone. Many of the subjects with ADRs (61.1%) had more than three concomitant pathologies and around 30% were taking more than seven drugs concomitantly. No statistically significant difference between males and females was found in all the above parameters of the CGA.
Table II illustrates the demographic and functional characteristics of patients divided according to the drugs most frequently involved in the ADR. Patients with ADRs related to the use of NSAIDs had a mean age of 75.0 ± 6.6 years, showed a prevalence of ADL and IADL disability of 17.4% and 31.6%, respectively, had a mean co-morbidity index of 2.5 ± 1.1 and were malnourished in >36% of cases. Patients with ADRs related to the use of aspirin (acetylsalicylic acid) had a mean age of 77.8 ± 8.1 years, showed a prevalence of ADL and IADL disability of 30.8% and 53.8%, respectively, had a mean co-morbidity index of 3.1 ± 1.2 and were malnourished in 33.3% of patients. Patients with a warfarin-related ADR showed an ADL and IADL dysfunction in 45% and 65%, respectively, 68.4% of them were malnourished and 47.6% lived alone. They also took a high number of drugs (mean = 6.9 ± 2.4). Patients with a digoxin-related ADR had a mean age of 84.8 ± 4.3 years, with a low educational level (3.8 ± 2.0 years), a high frequency of cognitive impairment (50%) and functional impairments in the ADL (41.7%) and IADL (91.7%). Moreover, 46.1% of these patients were affected by chronic renal failure.
Clinical Features of ADRs and Drugs Involved in the ADRs
Table III shows the clinical characteristics of the ADRs. The most frequent ADRs were gastrointestinal disorders (48 patients, 47.1%); platelet, bleeding and clotting disorders (20 patients, 19.6%); and cardiovascular disorders (13 patients, 12.7%). The drugs most frequently involved as causes of ADRs were NSAIDs (23.5%), warfarin (20.6%), low-dose aspirin (13.7%), digoxin (12.7%), amiodarone (3.9%), ACE inhibitors (3.9%), analgesics (2.9%) and antibiotics (2.9%). All others were responsible for <5% of the total ADRs. The ADR-related gastrointestinal disorders were mainly the result of the use of NSAIDs, low-dose aspirin, warfarin and dexa-methasone. Bleeding was the most common gastrointestinal ADR, having occurred in 16 (15.7%) patients. Peptic ulcer and erosive gastritis occurred in 0.8% and 9.8% of patients, respectively, and were the result of the use of an NSAID and/or low-dose aspirin. Digoxin was responsible for gastrointestinal symptoms such as nausea and vomiting in 9.8% of patients. Warfarin was the cause of 19.6% of ADRs, leading to subcutaneous haematomas (nine patients), haematuria (three patients), other haemor-rhagic lesions (cerebral, gingival or varix rupture) (three patients) and high INR values without bleeding (five patients). Cardiovascular disorders occurred in 12.7% of all ADRs and were associated ainly with anti-hypertensive drugs, i.e. agents acting on the renin-angiotensin system (ACE inhibitors and angiotensin II antagonists), diuretics or β-blocking agents.
Drug-Drug Interactions and Assessment of Avoidability of ADRs
Table IV includes the drug-drug interactions and their potential importance in inducing ADRs. Altogether, 33 of 102 ADRs (32.3%) showed a potentially relevant drug-drug interaction. In particular, 13 of 21 patients with a warfarin-related ADR (61.9%), six of 24 patients with an NSAID-related ADR (25%) and all 13 patients with a digoxin-related ADR (100%) had a drug-drug interaction that was potentially important.
According to Hallas et al.[19] and Gurwitz et al.[10] criteria, 45.1% of ADRs were defined as definitely avoidable, 31.4% as possibly avoidable, 18.6% as unavoidable and 4.9% as unclassifiable (figure 1). Of 78 patients with definitely avoidable or possibly avoidable ADRs, 17 patients (21.8%) had received an inappropriate prescription (disease or condition for which the drug was contraindicated, or prescription of a drug not indicated for the diagnosed disease), 29 patients (37.2%) had not received a prescription for an effective gastroprotective drug con-comitantly with NSAID or low-dose aspirin treatment and 32 patients (41%) were not monitored during treatment (table V).
Evaluating the relationship between avoidability of the ADR and a potentially important drug-drug interaction, seven patients with NSAID/low-dose aspirin and one patient with a digoxin-related definitely avoidable ADR (as a result of inappropriate prescription and/or no use of a prevention therapy) had a drug-drug interaction; moreover, 12 patients with a digoxin-related ADR and all 13 patients with a warfarin-related possibly avoidable ADR (as a result of inadequate monitoring) had a drug-drug interaction.
Discussion
We have undertaken a prospective analysis of ADRs as a cause of hospital admission in an elderly Italian population. Our data show that 5.8% of all admissions were related to ADRs. This finding is very similar to the 6.5% of ADR-related hospitalizations reported in a recent prospective study carried out in the UK,[8] and is quite similar to the cumulative ercentage of 6.7% reported in a meta-analysis of 39 prospective studies carried out in the US from 1966 to 1996.[25] None of these studies was performed specifically in elderly patients. Higher rates of ADR-related hospitalizations were reported in a meta-analysis of observational studies.[26] Of 7553 hospitalizations of elderly patients, 1251 (16.6%) were judged to be the result of an ADR-related problem. In that meta-analysis, larger studies found a lower prevalence of ADR-related hospitalizations, whereas smaller studies found a higher prevalence. A recent study carried out in elderly patients from Brazil [27] reported an 11.2% prevalence of ADR-related hospitalizations. Differences in definitions of ADR, data collection methods and target populations may account for these discrepancies. Indeed, most of the studies included in the meta-analyses were >20 years old, and it is disappointing that the rates of ADR-related hospital admissions have not decreased over this time.
In agreement with previous studies,[2–5,28] NSAIDs were identified as the cause of ADRs in 23.5% of all cases. The widespread use of these drugs for the treatment of osteoarticular diseases that are highly prevalent in old age probably accounts for this ADR rate. In this study, patients with an NSAID-related ADR had a mean age of 75 years, a low prevalence of disability (ADL = 17.4%, IADL = 31.6%), co-morbidity (2.5 ± 1.1) and poor nutritional status (36.8%), whereas a great number (83.3%) of patients were living within the family. Conversely, their far from adequate level of effective gastroprotection (20.8% of patients) was a disturbing finding and confirmed a recent Italian study that reported effective gastroprotective cover with NSAID use for only 13% of patients.[29] Two recent studies have demonstrated that treatment with proton-pump inhibitors reduces the risk of both uncomplicated peptic ulcer[30] and upper gastrointestinalbleeding[31] in elderly acute or chronic users of aspirin or NSAIDs. Because elderly patients are at higher risk of NSAID-related ADRs, strategies need to be implemented for the prevention of NSAID-related gastrointestinal disorders in this population.
Warfarin was implicated in 20.6% of ADRs. Oral anticoagulation in elderly patients is a dilemma. Although many elderly patients have strict indications for treatment with coumarin derivatives, increased bleeding risk with age is a matter of concern.[32] The risk of bleeding increases with the intensity of anticoagulation in a log-linear fashion, and recently it has been shown that INR is positively correlated with the risk of mortality.[33] In the present study, >60% of subjects with a warfarin-related ADR were dysfunctional in one or more ADL or IADL parameters, and >65% of patients were malnourished. Moreover, almost 50% of patients were living alone and taking a high number of drugs (mean of six medications per person). Although prescription of warfarin was judged to be appropriate in most patients, monitoring of therapy was often inadequate. On this point, several studies suggest that improvements in primary care monitoring may significantly reduce warfarin-related ADRs.[34,35] Recent studies report that nurse-led monitoring clinics,[35] computerized decision support systems,[34,35] patient education and involvement[36] and/or patient self-management[37] may all help to reduce the risk of ADRs.
Aspirin use was involved in 13.7% of ADRs. Low-dose aspirin for antiplatelet therapy in the prevention of cardiovascular or cerebrovascular disease is associated with a higher risk of ADRs.[38] Anti-platelet therapy with low-dose aspirin, i.e. 75–325 mg daily, reduces the risk of vascular-related death, non-fatal myocardial infarction and stroke in patients with previous myocardial infarction, unstable angina, non-haemorrhagic stroke or a transient ischaemic attack.[39,40] However, the use of low-dose aspirin is significantly associated with an increase in peptic ulcer and its complications, i.e. bleeding, particularly in elderly patients.[41,42] In the present study, five patients treated with low-dose aspirin had gastric bleeding, whereas two and six patients had peptic ulcer and erosive gastritis, respectively. The inadequate use of gastroprotective drugs in these patients may be the cause of the elevated frequency of these ADRs. Indeed, several studies have demonstrated that concomitant therapy with gastroprotective drugs reduces the risk of upper gastrointestinal bleeding in elderly patients. [30,31,43,44]
The issue of digoxin and digitalis toxicity was explored in 21 studies included in a systematic review, and digoxin overdose appeared to be the second leading cause of ADRs in five studies.[45] In the present study, 12.7% of ADRs were digoxin related. Patients with digoxin intoxication were older (mean age of 84.8 ± 4.3 years) and had a lower educational level. They were more disabled both functionally and cognitively and 90% of them were malnourished. Moreover, 46.1% of these patients were affected by chronic renal failure. It is evident that digoxin continues to be a drug that is difficult to manage and that its monitoring is necessary, particularly in elderly patients who are at high risk of decreased renal function.[46] Moreover, drug interactions, such as furosemide with digoxin, are frequent, and an advanced age >80 years is an independent risk factor for digoxin intoxication.[47] Indeed, monitoring of digoxin serum levels is still relatively uncommon in clinical practice. Unfortunately, in this study, the frequency of serological monitoring of digoxin was not evaluated.
In this study, a potentially important drug-drug interaction was reported in 32.3% of patients with ADR. This finding is in agreement with previous studies that evaluated the ADR prevalence in elderly hospitalized patients.[27,48] The most frequent interactions were observed in patients who had digoxin-or warfarin-related ADRs. On this point, it is important to stress that all patients with a digoxin- or warfarin-related possibly avoidable ADR as a result of inadequate monitoring had a potentially important rug-drug interaction. These data suggest that drug-drug interaction may play a major role in inducing a severe ADR and that a drug-drug interaction needs to be carefully evaluated, particularly in elderly subjects who need polypharmacy.
Another remarkable point of this study is the high rate of avoidable ADRs. Adopting the Hallas criteria, our data indicate that >75% of ADRs were either possibly (45.1%) or definitely avoidable (31.4%); this finding is in agreement with data from recent studies in the UK[8,49] and US (63–67%). Analysis and categorization by type of error and outcome suggested that three high-priority preventable ADRs accounted for 75% of all reports: (i) the lack of ffective gastroprotection when treated with NSAID or low-dose aspirin was associated with gastrointestinal bleeding, peptic ulcer and erosive gastritis (29 patients); (ii) insufficient monitoring and adjustment of anti-coagulant dose according to laboratory test values was associated with haemorrhagic events in 13 patients; and (iii) overdoses of digoxin were associated with vomiting, nausea and cardiac disorders (12 patients). Medical error, i.e. inappropriate prescription of drugs, or failure to prescribe protective drugs, was the most frequent cause of definitely avoidable ADRs.
Conclusion
In conclusion, ADRs, a significant cause of hospital admissions, demand increased pharma-covigilance from all in the health services field. More important, a considerable percentage of ADRs are avoidable. Further studies aiming to evaluate the usefulness of prevention strategies in specific subgroups of patients at high risk of ADRs need to be implemented, particularly in the elderly population.
References
Pilotto A, Franceschi M, Vitale D, et al. on behalf of FIRI and SOFIA Project Investigators. Drug use by the elderly in general practice: effects on upper gastrointestinal symptoms. Eur J Clin Pharmacol 2006; 62: 65–72
Onder G, Pedone C, Landi F, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc 2002; 50: 1962–8
Beyth RJ, Shorr RI. Epidemiology of adverse drug reactions in the elderly by drug class. Drugs Aging 1999; 14: 231–9
Mannesse CK, Derkx FH, de Ridder MA, et al. Adverse drug reactions in elderly patients as contributing factor for hospital admission: cross sectional study. BMJ 1997; 315: 1057–8
Cunningham G, Dodd TRP, Grant DJ, et al. Drug-related problems in elderly patients admitted to Tayside hospitals, methods for prevention and subsequent reassessment. Age Ageing 1997; 26: 375–82
Mannesse CK, Derkx FHM, De Ridder MAJ, et al. Contribution of adverse drug reactions to hospital admission of older patients. Age Ageing 2000; 29: 35–9
Doucet J, Chassagne P, Trivalle C, et al. Drug-drug interactions related to hospital admission in older adults: a prospective study of 1000 patients. J Am Geriatr Soc 1996; 44: 944–8
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. BMJ 2004; 329: 15–9
Ghandi TK, Burstin HR, Cook F, et al. Drug complication in outpatients. J Gen Intern Med 2000; 15: 149–54
Gurwitz JH, Field TS, Harrold LR, et al. Incidence and prevent-ability of adverse drug events among older persons in the ambulatory setting. JAMA 2003; 289: 1107–16
Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing home. Am J Med 2000; 109: 87–94
Winterstein AG, Sauer BC, Hepler CD, et al. Preventable drug-related hospital admissions. Ann Pharmacother 2002; 36: 1238–48
Rubenstein LZ, Joseph T. Freeman award lecture: comprehensive geriatric assessment: from miracle to reality. J Gerontol A Biol Sci Med Sci 2004; 59: 473–7
L’informatore farmaceutico 2004. OEMF International, Milano 2002. Guidelines for ATC-Classification. Uppsala: NLN-publication No.16 Nordic Council on Medicines, 1985
Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255–9
World Health Organization. International drug monitoring: the role of national centres. WHO technical Report series. 498. Geneva: World Health Organization, 1972
Thomson Micromedex Healthcare Series [online]. Available at URL: http://www.thomsonhc.com [Accessed 2007 Aug 21]
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–45
Hallas J, Harvald B, Gram LF, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med 1990; 228: 83–90
Katz S, Downs TD, Cash HR, et al. Progress in the development of an index of ADL. Gerontologist 1970; 10: 20–30
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–86
Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975; 23: 433–41
Linn B, Linn M, Gurel L. The cumulative illness rating scale. J Am Geriatr Soc 1968; 16: 622–6
Guigoz Y, Vellas B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme 1999; 1: 3–11
Lazarou J, Pomeranz BH, Corey PN, et al. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279: 1200–5
Beijer HJM, Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 2002; 24: 46–54
Passarelli MC, Jacob-Filho W, Figueras A. Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause. Drugs Aging 2005; 22: 767–77
Vila A, San Jose A, Roure C, et al. Prospective multicenter study of adverse drug in hospitalized elderly patients. Med Clin 2003; 120: 613–8
Pilotto A, Franceschi M, Vitale DF, et al. on behalf of F.I.R.I. (Fondazione Italiana Ricerca sull’Invecchiamento and the SOFIA Project Investigators). Upper gastrointestinal symptoms and therapies in elderly out-patients, users of non-selective NSAIDs or coxibs. Aliment Pharmacol Ther 2005; 22: 147–55
Pilotto A, Franceschi M, Leandro G, et al. Proton-pump inhibitors reduce the risk of uncomplicated peptic ulcer in elderly either acute or chronic users of aspirin/non steroidal anti-inflammatory drugs. Aliment Pharmacol Ther 2004; 20: 1091–7
Pilotto A, Franceschi M, Leandro G, et al. The risk of upper gastrointestinal bleeding in elderly acute and chronic users of aspirin and other non-steroidal antiinflammatory drugs: the role of gastroprotective drugs. Aging Clin Exp Res 2003; 15: 494–9
Torn M, Bollen Ward LEM, van der Meer FJM, et al. Risk of oral anticoagulant therapy with increasing age. Arch Intern Med 2005; 165: 527–32
Oden A, Fahlen M. Oral anticoagulation and risk of death: a medical record linkage study. BMJ 2002; 325: 1073–5
Fihn SD, McDonell MB, Vermes D, et al. A computerised intervention to improve timing of outpatient follow-up: a multicenter randomised trial in patients treated with warfarin. J Gen Intern Med 1994; 9: 131–9
Fitzmaurice DA, Hobbs FD, Murray ET, et al. Oral anticoagulation management in primary care with the use of computerized decision support and near-patient testing: a randomised, controlled trial. Arch Intern Med 2000; 160: 2343–8
Sawicki P. A structured teaching and self-management program for patients receiving oral anticoagulation: a randomized controlled 556 trial. Working group for the study of patient self-management of oral anti-coagulation. JAMA 1999; 281: 145–50
Cromheecke M. Oral anticoagulation self management and management by a specialist anticoagulant clinic: a randomized cross-over comparison. Lancet 2000; 356: 97–102
Pilotto A, Franceschi M, Leandro G, et al. Geriatric Gastroenterology Study Group (Societe Italiana Gerontologie Geriatria). NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging 2003; 20: 701–10
Antiplatelet Trialist’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy. I. Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994; 308: 81–106
The SALT Collaborative Group. Swedish Aspirin Low-dose Trial (SALT) of 75 mgr aspirin as secondary prophylaxis after cerebrovascular events. Lancet 1991; 338: 1345–53
Weil J, Colin Jones D, Langman MJS, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ 1995; 310: 827–30
Serrano P, Lanas A, Arroyo MT, et al. Risk of upper gastrointestinal bleeding in patients taking low-dose aspirin for the prevention of cardiovascular diseases. Aliment Pharmacol Ther 2002; 16: 1945–53
Pilotto A, Franceschi M, Longo MG, et al. Helicobacter pylori infection and the prevention of peptic ulcer with proton pump inhibitors in elderly subjects taking low-dose aspirin. Dig Liver Dis 2004; 36: 666–70
Pilotto A, Franceschi M, Leandro G, et al. Helicobacter pylori infection and the risk of gastro-duodenal damage in symptomatic elderly chronic low-dose aspirin users: effect of antisecretory drugs. Age Ageing 2004; 33: 402–4
Wiffen P, Gill M, Edwards J, et al. Adverse drug reactions in hospital patients: a systematic review of the prospective and retrospective studies. Bandolier Extra, June 2002 [online]. Available at URL: http://www/jr2.ox.ac.uk/bandolier/extra.html [Accessed 2007 Aug 27]
Corsonello A, Pedone C, Corica F, et al. Gruppo Italiano di Farmacovigilanza nell’Anziano (GIFA) Investigators. Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients. Arch Intern Med 2005; 165: 790–5
Pahor M, Guralnik JM, Gambassi G, et al. The impact of age on risk of adverse drug reactions to digoxin. For The Gruppo Italiano di Farmacovigilanza nell’ Anziano. J Clin Epidemiol 1993; 46: 1305–14
Juurlink DN, Mamdani M, Koop A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA 2003; 289: 1652–8
Howard RL, Avery AJ, Howard PD, et al. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Health Care 2003; 12: 280–5
Acknowledgements
This work was supported by grants from the Italian Ministry of Health, IRCCS Research Program 2006–2008, Line 2: ‘Malattie di rilevanza sociale’. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franceschi, M., Scarcelli, C., Niro, V. et al. Prevalence, Clinical Features and Avoidability of Adverse Drug Reactions as Cause of Admission to a Geriatric Unit. Drug-Safety 31, 545–556 (2008). https://doi.org/10.2165/00002018-200831060-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200831060-00009