Article Text
Abstract
Functional constipation (FC) is a common medical problem in children, with minimal risk of long-term complications. We determined that a large number of children were being admitted to our children’s hospital for FC in which there was no neurological or anatomical cause. Our hospital experienced a patient complication in which a patient died after inpatient treatment of FC. Subsequently, we developed a standardised approach to determine when paediatric patients needed hospitalisation for FC, as well as to develop a regimented outpatient therapeutic approach for such children to prevent hospitalisation. Our quality improvement initiative resulted in a large decrease in the number of children with FC admitted into the hospital as well as a decrease in the number of children needing faecal disimpaction in the operating room. Our quality improvement process can be used to decrease hospitalisations, decrease healthcare costs and improve patient care for paediatric FC.
- pediatric
- functional
- constipation
- hospitalization
- quality
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Problem
The medical care of patients presenting with constipation to our children’s hospital is an area that we felt needed to be improved. A large number of children were being admitted to the inpatient service with a diagnosis of ‘constipation’ for which the majority of these children had no neurological or anatomical cause, and thus would be defined as having ‘functional constipation’ (FC) per Rome Criteria (http://www.romecriteria.org/assets/pdf/19_RomeIII_apA_885-898.pdf). Additionally, different therapeutic regimens were being used in our hospital to treat constipation, and such regimens were not standardised. As a result, there was wide variability in hospital length of stay as well as clinical outcomes for such children, leading to issues related to patient discomfort, patient risk, medical costs, and a mixed message given to patients, their families and the medical community. We describe a standardised approach that our hospital developed to improve the medical care of paediatric patients with FC in order to decide when paediatric patients needed hospitalisation for FC, as well as to develop a standardised outpatient therapeutic approach for such children to prevent hospitalisation.
Background
FC is a common disorder, affecting up to 3% of children worldwide.1 FC accounts for an economic impact of $3.9 billion of healthcare spending in the USA alone. The psychosocial burden of FC to patients and their families is substantial, with excess school and work absenteeism, as well as social isolation and bullying related to encopresis.2
FC has a behavioural origin in most children and is typically benign with minimal risk of long-term clinical sequelae.3 The pathophysiology of FC involves painful bowel movements, leading to faecal withholding and a toileting fear. Large, withheld stools ultimately cause more pain, leading to more withholding, creating a vicious cycle of disordered defaecation and bathroom avoidance. If untreated, this problem progresses to rectal dysfunction and incomplete rectal emptying, learnt avoidance of the normal urge to void, and recurring involuntary faecal incontinence. FC is easily managed in the outpatient setting in most children. The mainstays of therapy are parental education, scheduled oral osmotic laxative therapy (such as lactulose or polyethylene glycol 3350 (PEG3350)) to soften stool, and home behavioural modification programmes involving scheduled toilet sitting typically after meals. Oral ‘bowel cleanouts’ using large volume or increased dosing of osmotic laxatives are effective for most patients with refractory FC.
Despite effective outpatient management options, many children with FC are admitted to the hospital setting for nasogastric tube administration of osmotic laxatives, particularly those children seen in an emergency department setting. A large percentage of such patients (40%) receive no outpatient constipation therapy prior to hospital admission.4 Thus, structured laxative therapy programmes potentially can reduce hospital admissions of children with normal anorectal anatomy.5 Additionally, inappropriate use of specific laxative regimens for constipation has been associated with morbidity and mortality.6 7 A prior quality improvement initiative from Boston Children’s Hospital, which provided clinical guidelines for paediatric constipation treatment for primary care physicians, did lengthen the time before referral to a paediatric gastroenterologist, although it did not reduce the total number of referrals to a paediatric gastroenterologist for constipation.8
At our institution, we noted a significant number of otherwise healthy paediatric patients admitted to our hospital with FC solely for therapeutic management (‘bowel cleanout’). An analysis of Pediatric Health Information System (PHIS) (https://www.childrenshospitals.org/programs-and-services/data-analytics-and-research/pediatric-analytic-solutions/pediatric-health-information-system) data revealed that our hospital system had the highest rate of inpatient bowel cleanout admissions from the emergency department for children with FC compared with other PHIS member hospitals in the USA. Additionally, our hospital had a wide array of constipation treatment regimens for both the inpatient hospital service and emergency department that were not standardised. We then had a sentinel event at our institution in 2011 in which a healthy child with FC died in the hospital due to a complication of a milk and molasses enema. These data and this specific event led to a system-wide reanalysis of our FC management practices and a push to manage more of these children as outpatients.
Our paediatric gastroenterology and emergency medicine divisions previously developed and implemented quality improvement initiatives to standardise management and to reduce hospitalisations for FC. Patients in the emergency department were supplied discharge kits containing home bowel cleanout instructions, a large plastic cup, drink flavouring powder and osmotic laxative medication at standardised doses. We completed an educational campaign in our gastroenterology and emergency medicine divisions regarding published research demonstrating the lack of utility of abdominal radiographs to diagnose FC.9 Abdominal radiography utilisation decreased and patient satisfaction increased, but hospital admission rates for FC did not change substantially.
We proceeded to create a standardised treatment algorithm for FC with a goal of improving therapy for FC in our paediatric emergency department, as well reducing the number of admissions to the hospital for FC. The algorithm had two components as follows: (1) development of a better communications system between the emergency department and our paediatric gastroenterologists for any patient with FC who was being considered for admission, with the development of standardised inpatient orders for constipation treatment; and (2) development of an outpatient therapy algorithm to prevent hospital admissions for FC.
Measurement
After determining that we admitted more patients with FC from the emergency department than other children’s hospitals with similar acuity and volume, we decided to interview paediatric community physicians as well as our hospital’s paediatric gastroenterologists, paediatric hospitalists, paediatric surgeons and emergency department physicians to understand the culture of admitting patients to the hospital for FC. We also reviewed the medical records of over 100 inpatient admissions for FC prior to 2015. Several recurring themes surfaced, which are summarised in the box. We then targeted interventions to correct physician knowledge deficits and physician misconceptions.
Problem list for hospital culture of admitting paediatric patients for FC prior to intervention (defined as either ‘Physician Knowledge Deficit’ or ‘Physician Misconception’
Physician knowledge deficit
There was little to no familiarity with established paediatric gastroenterology society practice guidelines for constipation (North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition or NASPGHAN).
Many patients admitted to the hospital for FC had never tried any home laxative therapy.
There was no definition of a standard enema composition or volume. A wide variation of enema volume for patients with the same weight as well as multiple types of enemas were used.
The term ‘fecal impaction’ had no standard definition. Most abnormal digital rectal exam findings were labelled as ‘fecal impaction’, and this term was frequently cited as a ‘red flag’ necessitating admission to the hospital.
The terms ‘fecal impaction’ and ‘bowel obstruction’ were interchangeably used when there was no other physical exam finding or radiographical imaging evidence of an intestinal obstruction (eg, progressive abdominal distension, vomiting, abdominal radiography showing fluid–air levels).
Patients who had an initial hospitalisation for a ‘bowel cleanout’ were automatically readmitted for any subsequent FC events without consideration of outpatient therapy first.
Patients with FC and ‘fecal impaction’ frequently were going to the operating room for a faecal disimpaction with no signs of bowel obstruction on physical examination or findings of obstruction on radiographical imaging.
Physician misconception
There was no standard definition of a ‘bowel cleanout’ at home. There was no minimum dosage of ingested laxative medication. Patients with underdosed ‘cleanouts’ (eg, 17 g of PEG3350 twice daily for 2 days in a 60 kg teenager) were admitted under the presumption that a ‘cleanout’ had already been tried and failed.
Patients subjectively were assessed to ‘not be able to drink’ the necessary volume of laxatives for a home cleanout attempt and were admitted before trying. Providers had a belief that typical outpatient bowel cleanout regimens were ‘too difficult’ or ‘impossible’ for most paediatric patients to ingest.
Digital rectal examination findings were interpreted in ways that were not clinically meaningful. Terms such as ‘stool ball’, ‘fecaloma’, ‘stool mass’ and so on were subjectively determined to be ‘too large’ or ‘too hard’ to pass, although no such evidence-based assessment exists.
There was a belief that enteral laxative therapy could not be used in the setting of ‘fecal impaction’. If stool could not be cleared manually, first, subsequent enteral laxative use was perceived as a risk factor of progression to bowel obstruction or bowel perforation.
There was a belief that enema therapy for ‘fecal impaction’ would lead to bowel perforation or death.
There was a belief that outpatient therapy for FC could often be fatal (after the sentinel event of mortality occurred in our patient who was hospitalised for constipation treatment).
Community providers were sending patients to our emergency department for a ‘bowel cleanout’ without any laxative therapy tried as an outpatient first.
Abdominal radiography was being used as a sole means to subjectively label patients as having a ‘stool ball’ that was ‘too large to pass’, regardless of no evidence of bowel obstruction and no correlation with clinical history or physical exam findings.
FC, functional constipation; PEG3350, polyethylene glycol 3350.
Design
We started our intervention with a global consensus on how to prevent hospitalisation for paediatric FC and to address physician knowledge deficits and physician misconceptions (box). Our intervention consisted of the Plan-Do-Study-Act (PDSA) cycles (https://innovations.ahrq.gov/qualitytools/plan-do-study-act -pdsa-cycle). Thus, PDSA cycle 1 consisted of required physician-to-physician communication between the paediatric emergency department (or referring outpatient provider or outside facility in the case of requests for direct admission to the hospital) and the on-call paediatric gastroenterologist for any potential hospital admission for FC in order to assist in patient triage. The goals of this intervention were to prevent hospital admission by encouraging outpatient laxative therapy (for home) prior to hospital admission, to provide education about FC to prevent hospital admissions based solely on radiographical findings or digital rectal exam findings, and to offer quick follow-up (within 24–48 hours) in our paediatric gastroenterology clinic if needed. Additionally, PDSA cycle 1 consisted of creating a standardised inpatient order set for our children’s hospital for treatment of FC not amenable to the outpatient algorithm. The main purpose of this order set was to limit the volume of PEG3350 given over a 24-hour period. These orders were clear that patients could receive up to 80 mL/kg of PEG3350 via the oral or nasogastric route up to a maximum volume of 4 L (adult maximum volume). High-volume PEG3350 was required to be given over 10 hours to prevent complications such as renal injury.10
We then progressed to PDSA cycle 2, which was the development of a standard triage algorithm to assist outpatient healthcare providers in the treatment of FC (figure 1) to further address physician knowledge deficits and physician misconceptions (box). This algorithm would be required prior to consideration of hospital admission for constipation management. The algorithm specifically stated that only a visual inspection of the anus was required for patients with FC with no other significant medical history prior to initial enema administration due to our finding that digital rectal examination findings often were misinterpreted in the emergency department and outpatient setting. Thus, we did not recommend a digital rectal examination or disimpaction in this setting. The algorithm required use of at least two large-volume normal saline enemas prior to hospital admission.
Outpatient algorithm for treatment of FC in children. ED, emergency department; PEG3350, polyethylene glycol 3350; RTU, rapid treatment unit.
We developed a standard enema protocol for use in both the emergency department and inpatient setting for treatment of FC not amenable to oral laxative therapy. Enemas would consist of room temperature normal saline given at a volume of 20 mL/kg up to a maximum volume of 1 L. The addition of 50 mL of glycerin was added to the enema if patients weighed over 50 kg. The enema protocol consisted of a retention technique in which an 8-French balloon Foley catheter was lubricated and gently inserted in the rectum. Normal saline was given through the catheter with the patient retaining the enema for 10 min, when possible. An experienced colorectal clinic nurse practitioner led training of emergency department and inpatient nursing staff in this technique. Passage of ‘brown’ output per rectum was determined to be successful and did not require hospital admission for constipation management. The algorithm specifically allowed patients with good transportation options to go home from the emergency department if the patient or family wanted to retain the enema and wait for enema results at home. Milk and molasses enemas were not allowed due to the mortality risk.6 Patients who then proceeded to the ‘Appropriate Home Cleanout’ aspect of the algorithm were instructed to follow a clear liquid diet during the cleanout, although we did not assess compliance with this diet.
Strategy
Our proposed algorithm had two aspects: (1) ensuring better communication between the emergency department and our paediatric gastroenterologists for any patient with FC who was being considered for admission and use of standardised inpatient orders for those patients who required admission for treatment of FC as described above (PDSA cycle 1), and (2) use of an outpatient therapy algorithm to prevent hospital admissions for FC (PDSA cycle 2) (figure 1). PDSA cycle 1 was started 12 months prior to starting cycle 2 and continued during implementation of cycle 2.
Results
PDSA cycle 1
PDSA cycle 1 started in June 2015 and data recording occurred biannually for 24 months. In order to determine the effectiveness of this protocol, we reviewed the records of all paediatric patients seen in our emergency department for FC. If patients were seen for FC, we evaluated why such patients were admitted into the hospital and if they were hospitalised for a ‘bowel cleanout’. We further categorised these admitted patients as ‘constipation-healthy’ or ‘constipation-not healthy’. ‘Constipation-not healthy’ was defined as having a concomitant diagnosis of cerebral palsy, neuromuscular disease, anorectal malformation, Hirschsprung disease, or severe autism spectrum or psychiatric disorder with documented violent behaviour. Hospital admissions for FC were further defined as ‘appropriate’ or ‘not appropriate’. Admissions were considered not appropriate if they included scenarios in which a patient was admitted into the hospital without an attempt of using an oral bowel regimen programme or without using a normal saline enema in the emergency department in the setting of haemodynamic stability and no signs or symptoms of a bowel obstruction. All other admissions for FC were considered appropriate. We then compared the total number of FC admissions with the overall volume of patients with FC seen and discharged from our emergency department. We also determined the total number of patients undergoing a manual faecal disimpaction in the operating room area of our children’s hospital.
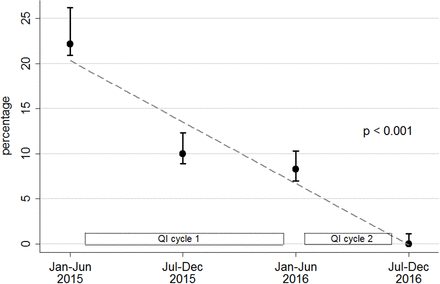
In the first two biannual measurements of our intervention, we saw a large decrease in the number of healthy children with FC admitted to the hospital who had not undergone either an oral laxative cleanout regimen at home or a normal saline enema in our emergency department. Initially 10.1% of patients with FC were admitted from our ER without trying outpatient laxative therapy or a normal saline enema in the emergency department, but this percentage decreased to 3.6% (figure 2) within 6 months. Only a small decrease initially was observed in the number of children who had an inappropriate admission for FC and who subsequently underwent a manual faecal disimpaction in the operating room (figure 3).
Graph demonstrating percentage of children with functional constipation (constipation-healthy) admitted to the hospital without a prior home laxative regimen or use of a normal saline in the emergency department. Data divided into biannual measurements. A significant decrease in the number of children admitted into the hospital was noted at the end of the study (p=0.013 by Poisson regression).
Graph demonstrating percentage of children with functional constipation (constipation-healthy) who underwent faecal disimpaction in the operating room during hospital admission. Data divided into biannual measurements. A significant decrease in the number of children requiring faecal disimpaction in the operating room was noted at the end of the study (p<0.001 by Poisson regression).
PDSA cycle 2
This cycle started in 2016 (12 months after initiation of PDSA cycle 1) and was studied for 12 months with measurements obtained biannually. PDSA cycle 2 consisted of the continuing intervention of PDSA cycle 1 with the addition of an outpatient triage algorithm for treatment of FC in order to prevent hospital admission (figure 1). Thus, the hospital would be aware if such patients had ‘tried everything and failed’ before consideration for hospital admission. Although there was a slight initial increase in the percentage of healthy children with FC admitted to the hospital without prior use of a home oral laxative regimen or normal saline enema use in the emergency department, the final percentage of such children admitted into the hospital was significantly decreased (p=0.013) and was the lowest of the entire intervention at 1% (figure 2). We noted a significant decline (p=0.001) in the number of healthy children with FC undergoing manual faecal disimpaction as part of the hospital admission, with no children requiring this intervention at the end of PDSA cycle 2 (figure 3).
Lessons and limitations
Our intervention to prevent admissions of healthy children with FC was successful, with a dramatic decrease in the number of such children admitted into the hospital for treatment of FC. Additionally, we reduced the number of healthy children undergoing manual faecal disimpaction in the emergency department by using a standardised outpatient oral laxative regimen with the potential addition of serial normal saline enemas, if needed. We have continued to follow such patients over time and have seen a continuing low percentage of healthy patients admitted for FC, suggesting long-term success in our protocol. Our paediatric gastroenterology division recently has tried to improve outreach education as well for treatment of such children by making the outpatient protocol available to referral physician groups, and we have begun to give outreach lectures to outside physician groups to teach them about paediatric FC and treatment options. Additionally, we have made a video about FC in children on YouTube for parents and providers that is public (https://youtu.be/pNagQup0Upg). We now provide a handout with links to this video (including a Quick Response or QR barcode for the video) during office visits in our clinic, and we are providing this handout to outside providers, patients and their families as part of our educational outreach.
There are limitations to this intervention. Studies in the treatment of paediatric FC are not clear due to study heterogeneity and lack of placebo-controlled trials. However, PEG3350 and sennosides appear to be a safe medication to date.11 12 In addition, our intervention required significant time commitment by members of the paediatric gastroenterology division as well as hospital administration to make sure standardised care for paediatric FC was continuously implemented. It will need to be determined if this degree of intervention will be required long term or if the time spent on educating our children’s hospital, the emergency department, outside referral clinics and patient families will decrease over time. Indeed, we hypothesise that the initial increase in the percentage of healthy children with FC admitted to the hospital at the implementation of PDSA cycle 2 may have been due to the new education aspects of our algorithm in the emergency department, which required time to be taught and used.
Conclusion
Our intervention, consisting of mandatory physician-to-physician communication with gastroenterology, standardisation of inpatient order sets, standardisation of normal saline enema use and standardisation of an outpatient algorithm for FC, was extremely effective in reducing hospital admission of healthy children with FC. Our hospital’s goal is to keep the majority of such children out of the hospital long term through continuing data monitoring and education of our hospital staff, emergency department and outside referring physician groups. We also are hopeful that our experience will be used by other paediatric hospitals to decrease hospitalisations, decrease healthcare costs and improve patient care.
Footnotes
Competing interests None declared.
Ethics approval University of Utah Institutional Review Board; Primary Children’s Hospital Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.