Article Text
Abstract
Background Medication errors are an emerging problem in various hospital settings, especially in neonates. A study conducted in the neonatal care unit of a tertiary institute in Kolkata as baseline over 3 months, revealed total error to be around 71.1/100 prescriptions (median medication error percentage: 63%).
Purpose To assess the occurrences of medication errors and determine efficacy of Point-of-Care Quality improvement (POCQI) model in reducing the same from baseline 63% to less than 10%, in the above setting within next 9 months.
Materials and methods This quality improvement initiative of quasi-experimental design comprised randomly selected prescriptions and monitoring sheets of neonates admitted in the neonatal care unit, obeying inclusion and exclusion criteria. Medication errors were assessed and categorised using a predesigned and pretested checklist. Interventions were planned after forming a quality improvement team in four plan–do–study–act (PDSA) cycles spanning over 6 weeks each (including training of doctors and nurses, signature and countersignatures of respective healthcare personnel, computer-generated prescriptions and newly designed software-generated prescriptions) as per POCQI model of the WHO and results in post-intervention phase (3 months) were compared.
Results A total of 552 prescriptions and monitoring sheets of 124 neonates were studied. Median medication error percentages in first, second, third and fourth PDSA cycle were, respectively, 48%, 42%, 30% and 14%. Total error reduced to 10.4/100 prescriptions (p<0.005), with significant reduction in erred dosage, timing, interval, preparation and rate of infusion of drugs in prescriptions of the post-intervention phase.
Conclusion Implementation of change ideas via PDSA cycles, as per the POCQI model with technological aid, significantly decreased the percentage of medication errors in neonates, which was also sustained in the post-intervention phase and facilitated error-free prescriptions.
- Paediatrics
- PDSA
- Quality improvement
- Medical error, measurement/epidemiology
Data availability statement
Data are available upon request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
As documents furnished by the Institute Of Medicine, 44 000–98 000 deaths occur annually in the USA due to medical errors, which are emerging as serious problems in the healthcare system.1 Medication errors are by far the most common forms of medical errors in this era. The National Coordinating Council for Medication Error Reporting and Prevention defines medication error as any preventable event that may cause or lead unfitting medication use or induce damage to a patient while in procedures, prescribing medicines, communication of orders, labelling and packaging of products, dispensing distribution, administration and even while monitoring.2 Neonates pose particular challenges to medical experts in comparison with the adults.3 Rapidly changing body size parameters, off-label drug usage, evolving physiological systems affecting drug absorption, distribution, metabolism and excretion put them at a greater risk of harm from pharmaco-therapeutic interventions.4
Various studies conducted worldwide have reported high incidence of medication errors in this age group. Kaushal et al reported an error rate of 5.5/100 prescriptions,5 Simpson et al had almost similar results of medication errors using critical incident or spontaneous incident reporting methods.6 Most of the studies identified a faulty prescription and an inappropriate dose as the common errors.7 Incidences of medication errors overall are quite common in India. A study conducted in hospitalised patients in northern India depicts incidences being around 26%, while another study conducted in Karnataka shows an error rate of 14%.8 9 The reported error rates vary depending on whether patients, prescriptions or specific medications are used as the denominator. In 2004, a study of neonates in a tertiary care hospital in India done by Jain et al describes medication error rate to be 9.6 per 100 prescriptions, being significantly more in the neonatal emergency department.10
This study setting, a tertiary care centre of Kolkata, West Bengal, is a 64-bedded neonatal unit including a sick neonatal care unit (SNCU) and neonatal intensive care unit (NICU). It has an average monthly admission of around 300 babies including both inborn and referral cases. A total of 40 nursing personnel and 15 doctors toil round the clock to meet the needs of the ward. This, despite being a busy neonatal set-up, manages critical clinical cases with time and resource constraints. After daily morning rounds by the consultant doctors, directions for medications (advice) were updated through handwritten prescriptions in a definite format, maintained by the residents on duty, which were then copied in monitoring sheets by the nurses, keeping a record of drug administration. A baseline study conducted in this setting yielded a high medication error rate (71.1/100 prescriptions).
Studies depicting organised research over prevention of medication and prescription errors in both adults and the neonatal age group in the west have also documented methods of curbing down the error rate.11 Electronic medication prescribing, automated infusion devices and intensive education programmes were mostly tried for, by separate researchers targeting primarily prescription fallacies. This study addresses the alarming scenario here, especially in eastern India where both the data on prevalence of medication errors and any measures for their prevention, are lacking.
The WHO/SEARO (World Health Organization- South East Asia Region Office) collaborating with other institutions has provided the Point-Of-Care Quality Improvement (POCQI) model12 for analysis of practice performance, offering solutions to problems hindering delivery of standard healthcare. Various pioneer healthcare set-ups are applying this methodology to address the problems they are facing, involving maximal utilisation of available resource. Using this novel approach, this study aimed to assess and categorise the medication errors and reduce the same from a baseline median medication error percentage of 63% to less than 10% in the above setting within the next 9 months.
Methodology
This quasi-experimental study was conducted over a period of 1 year in the mentioned study setting. The study was done with randomly selected prescriptions and monitoring sheets of neonates admitted during this period, who received at least one oral or intravenous therapeutic drug among most commonly used drugs in SNCU (like intravenous fluids, antibiotics, methyl xanthines, anticonvulsants, inotropes, etc; topical and inhaled drugs, vaccines and vitamins were excluded). It was covered in three phases—the baseline phase of 3 months, an intervention phase of 6 months and a post-intervention phase of 3 months for verifying sustenance. Randomisation in each phase of the study was done using random number generation function of the MS Excel spreadsheet for selecting the prescriptions and monitoring sheets from different patients in different phases for evaluation. The prescriptions and monitoring sheets of those neonates who expired, transferred out, discharged or left against medical advice within 48 hours of admission were not included in the study due to indeterminate course and outcome.
Sample size for the baseline and post-intervention phase was calculated using a magnitude of medication error to be 9.6%,10 and an absolute error of 4%, making the count to 217 prescriptions. In the baseline phase, out of selected 217 prescriptions 37 had wrong or no patient particulars, time and date. These prescriptions with technical errors were considered separate and kept out of purview for calculation of medication errors. Hence, barring those, 180 prescriptions in the baseline phase and 212 prescriptions in the post-intervention phase were finalised.
Medication errors were categorised and defined as per the American Society of Hospital Pharmacists guidelines (table 1),13 which is also relevant in the Indian setting.10 A predesigned, pretested checklist was prepared for reviewing the prescriptions and monitoring sheets, which were subsequently checked for content validity by experts in the concerned subject. The demographic details were entered in the checklist from the case record sheets of the neonates whose prescriptions were selected and also from interviewing the mothers whenever possible after having their informed consent. Harriet Lane 21st edition was referred for all standard drug dosages, preparation and administration norms.14 Any deviation from the standard guidelines in respect to dose, interval, preparation, rate of infusion (rate of infusion not mentioned or prescribed at a slower or faster rate in case of anticonvulsants or antibiotics), delay in administration in case of emergency medicines (calculated by difference in prescription time and administration time recorded in monitoring sheet) over and above the allowable error margin was registered in the checklist (table 1). The final outcome (death/discharge) for the selected patients was also later traced and recorded.
Working definition of medication errors
A quality improvement (QI) team was formed according to POCQI module comprising a total of nine members including two resident doctors, two faculty members, three nursing staff and two sisters-in-charge, one resident doctor being the team leader. The team decided to conduct a meeting every week and collect prescriptions for review weekly. In the subsequent meeting, each member of the team presented one documented literature on the problem of the medication error and strategies for their resolution. One nursing personnel acted as a moderator of all the available literature, and record keeper of the change ideas and decisions taken. Another nursing personnel was made in charge for preparation and communication of time series charts. The team leader supervised that, time frame for data collection and analysis in each phase was maintained.
Measures
The outcome indicators that were used were, errors per 100 prescriptions, median medication error percentage in each phase, errors per prescription and mean errors per patient. Denominators used were the number of prescriptions in each phase of the study. For calculating mean errors per patient, the numbers of neonates recruited in each phase were used. As from baseline survey, it was seen that one prescription may have multiple errors, so error per prescription was used as an indicator. Prescription errors were henceforth used as a proxy indicator for medication errors with operational definitions listed in table 1. Various QI tools, like fish bone diagram and Pareto chart,15 were used to analyse the cause–effect scenario for the occurrences of medication errors. Time series charts were used to depict medication error percentage in each week, in each phase and median medication error percentages were calculated for comparison.
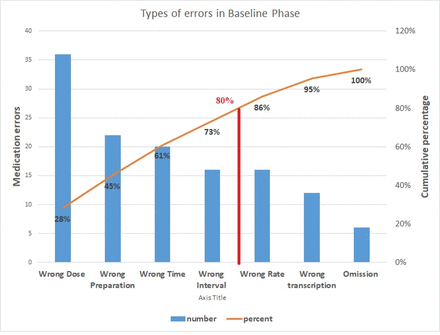
As mentioned earlier, a baseline survey was conducted over a span of 3 months with a total of 180 prescriptions and monitoring sheets of 32 neonates, and reviewed by independent members of the QI team. The treating doctors and nursing staff involved in management were unaware of the procedure and results. By cause–effect analysis with the help of a fish bone diagram (figure 1), the QI team identified certain lacunae which were as follows: (1) gap in knowledge regarding appropriate dosage; (2) there is no standard drug chart displayed for referring, while the doctors write prescriptions; (3) nursing staff have to copy the orders in the monitoring sheets daily, leading to errors while copying orders; (4) illegibility of the prescribed medicines in handwritten prescriptions; (5) lack of adequate electronic gadgets leading to dosage miscalculations while prescribing; (6) lack of accountability as complete legible signatures are absent in most prescriptions and monitoring sheets; (7) wrong interval of antibiotics which require changes as per the age of the neonate; (8) no system of cross-checking. A Pareto chart showed that major parts of the errors were wrong dose, wrong time, wrong preparation and wrong interval (figure 2).
Fish bone diagram showing cause–effect analysis of medication errors.
Pareto chart showing medication errors.
The QI team came up with some change ideas and tested them in a stepwise manner using the plan–do–study–act (PDSA) cycles, and implemented the successful PDSA cycles to achieve the aim. Each PDSA cycle was for 6 weeks and changes were implemented in prescriptions and monitoring sheets after daily morning rounds. All the successful changes experimented through one PDSA cycle were successively added up with the new idea for the next cycle.
The first PDSA cycle involved training of the on-duty doctors and nursing staff. The then posted health personnel were divided in two batches and each was trained in hour-long interactive sessions, two such in consecutive weeks, facilitated by the QI team members. Using audiovisual aids, they were acquainted with pharmacological properties, dosages, compatible preparations, interval and mode of administration of various drugs commonly used in the neonatal set-up. For their quick reference, a standardised drug list compiled from available pharmacology textbooks was prepared and handed over to them during training. This was also displayed in each working station of doctors and nurses for use during prescription and administration of drugs.14 The target of this PDSA was to minimise the wrong dose, wrong interval and wrong preparations of drugs. After the first PDSA cycle, there was marked decrease in the occurrence of medication errors especially those due to incorrect preparation of drugs. Hence, the changes in PDSA 1 were accepted and the system of prescribing medicines using the displayed drug list was made into practice. Still the scenario needed further improvement as wrong dosage and interval errors were not managed adequately (table 2).
Sociodemographic profile of the study subjects along with results of intervention in PDSA and post-intervention phase
The second PDSA cycle targeted to increase accountability for errors. It planned to ensure the prescriptions would contain full signatures of the doctors and monitoring sheets should have signatures of nursing staff. Senior faculty members were requested to go through daily morning shift prescriptions of babies admitted to nine NICU beds at that time and countersign them. The sister-in-charge would also cross-check respective patient charts (monitoring sheets) and countersign the medicines transcribed and administered by the nursing staff. This would initiate a system of cross-checking the drug dosages prescribed for those NICU patients and even any fallacy in transcribing or administering the medicines. The errors found, if any, in this procedure will be corrected instantly and informed privately to the undersigned resident and nursing staff. The QI team now intended to evaluate these double-checked prescriptions weekly after morning rounds selecting randomly, to look for any errors using the same checklist. Initial hurdle was motivating the residents and on-duty nursing personnel for mentioning their full signatures. Further procedural issues in obtaining prescriptions countersigned by the senior consultants led to delay in rolling over of the second PDSA. At the end of the second PDSA, the medication error percentages decreased to a considerable extent. Hence, the proposal for full signature and cross-checking with countersigning the prescriptions was accepted, yet the number was far from the smart aim targeted (ie, 10%). PDSA 2 also showed that decrease in dosage and interval errors still needed attention.
During the third PDSA cycle, a computer set-up was made available inside the neonatal ward and a resident was posted for preparing prescriptions for patients admitted to nine NICU beds in that period, after daily morning rounds by typing and calculating over the computer, thus aiming to minimise wrong calculation errors and illegibility. The QI team will evaluate some randomly selected prescriptions which were prepared over the computer weekly. The computer-typed prescription could not solve the problem of wrong interval and preparation of drugs as it was still dependent on the doctor for calculation of dosages and administration details of drug and moreover was time-consuming; there was rather an increase in occurrence of wrong dosage errors. Hence, the change idea for the PDSA 3 was abandoned as it was not feasible (table 2).
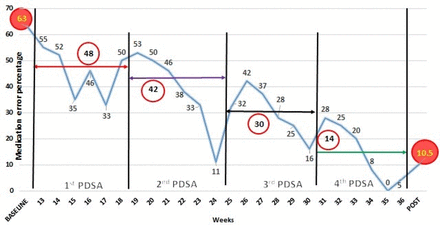
Easing the cumbersome process of manual computer entry, the final PDSA cycle introduced new software, ‘Neonate’, developed with technical support from software developers. The software was programmed with all the necessary prerequisite information of the commonly used drugs in the neonatal unit. One resident on duty would be assigned in the morning shift, who was already demonstrated the use of the software via the ongoing monthly training provided by the QI team. Prescriptions were generated as per decisions in the daily round initially, for the neonatal intensive care patients. For prescribing drugs using the software, first of all, a patient profile had to be created including date and time of birth, birth weight, current weight and other demographic details. After that, when the resident selected the desired drugs to be administered from the categorised drop-down list, appropriate dose was calculated automatically and administration details with interval and preparation were detailed. The final printed prescription would now contain all the necessary correct information. The QI team evaluated these computer-generated prescriptions in the said intervals as before. At the end of this fourth PDSA, wrong dose, wrong interval and wrong preparation errors were avoided. Weekly medication error percentage and median medication error percentage were calculated and displayed in time series charts throughout PDSA cycles to display the improvement ushered in (figure 3).
Time series chart of PDSA cycles with decline in median medication error percentage (circled figures), in comparison with the baseline and post-intervention phase. PDSA, plan–do–study–act.
During the successive 3 months (12 weeks of post-intervention phase), the QI team reviewed random post-morning round prescriptions for monitoring whether or not the changes brought in by the PDSA cycles, like using automated software installed in the computer while prescribing, full signature of the doctors and nursing staff in prescriptions and monitoring sheets, cross-checking and countersignature by respective senior members, were sustained throughout. The QI team also conducted monthly training of the doctors and nursing staff regarding appropriate use of drugs and utilisation of software for generating prescriptions due to monthly change in staffing pattern. On posting of new residents once in every 3 months, a detailed training session was held, mentored by the members of the QI team as well as the leaving batch of residents. Weekly meetings of the team continued as per availability of all members and prescriptions reviewed and discussed further on maintenance of the implemented changes.
Statistical analysis
Data were entered in Microsoft Excel spreadsheets and represented via tables and diagrams. Statistical analysis was done by using SPSS for Windows V.16 software. Comparisons of proportions were made using Χ2 test.
Results
A total of 552 prescriptions and monitoring sheets of 124 neonates were included in the study over the three phases. Demographic characteristics of the subjects whose prescriptions and monitoring sheets were studied are listed in table 2. In this study, overall, 58% were male and 42% were female, majority were preterm, suggesting more admission of preterm neonates in the unit. The mean overall gestational age was 34.5+3.32 weeks (range: 28−39 weeks) and mean birth weight was 1905+737 g (830−3900 g). It was also revealed that about 17% of prescriptions initially had technical errors like wrong or missing patient credentials and/or no mention of time and date. These errors were only found in 5 prescriptions out of 217 in the post-intervention phase.
In the baseline phase, the medication error percentage was found to be 71.1/100 prescriptions (95% CI: 64.5% to 77.7%), median medication error percentage being 63%. Maximum proportion was contributed by the wrong dosage errors (20%). Mean errors per neonate in the baseline phase amounted to 4.06+3.04.
With interventions in each PDSA cycle, the successive reduction in total errors and mean error per patient is shown in table 2.
The median prescription error percentage reduced to 48% in PDSA 1, 42% in PDSA 2, 30% in PDSA 3 and finally 14% in PDSA 4, which is also represented through time series charts (figure 3). Dosage, timing and preparation errors decreased from 18%, 11.4% and 6.8% in PDSA 1 to nil in PDSA 4, where only errors comprised interval miscalculations and transcribing errors, 5.4% each. Interval miscalculation, when analysed, was due to entry of incorrect credentials in the software.
Total errors significantly reduced to 10.4/100 prescriptions (95% CI: 6.9 to 15.2) and median medication error percentage being 10.5% in the post-intervention phase (figure 3). The mean errors per patient in the post-intervention phase reduced to 0.58+0.76.
Table 3 shows significant diminution in dosage, interval, timing and preparation errors in the post-intervention phase in comparison with the baseline phase. Transcription errors though showed no significant reduction. While in the baseline phase, 41.1% prescriptions had a single error and 13.3% had multiple errors/prescription, in the post-intervention phase, 10.4% of prescriptions had a single error/prescription only, depicting a significant reduction (p<0.01) (table 3).
Comparison of total and different types of errors in the baseline and post-intervention phase
Time series charts for comparison of the baseline and post-intervention phase were also drawn (figure 4). The occurrences of total errors or errors per prescription in the baseline and the post-intervention phase were not significantly correlated with gender or gestational age of the babies, whose prescriptions were included in the study (p=0.2; p=0.546). It was observed that in the baseline phase, 63.4% of the babies in the study purview were successfully discharged from hospital. Though the post-intervention phase showed a higher discharge rates of the concerned babies from the hospital (75.5%), this change was not significantly related to the reduction in total errors or errors per prescription (p=0.21).
Time series chart in the baseline and post-intervention phase.
Discussions
This project could ultimately achieve the smart aim of bringing down the median medication error percentage to around 10% with cumulative application and implementation of the ideas at the end of the four PDSA cycles. This is first of its kind in eastern India where the medication errors in neonatal set-up were documented and categorised and QI methodology was used along with a newly designed computer-based system for writing prescriptions.
Here the initial type of errors were technical, with erroneous entry of patient particulars, time and date amounting to 17% in the baseline phase. Reports document similar sort of errors to happen in other set-ups at similar rates.16 Eventually at the post-intervention phase, we could also improvise on this area as a software-generated prescription automatically includes time and date. As the main bulk of errors was due to dosage miscalculations, wrong preparation and interval errors, these were addressed primarily.
The occurrence of total errors in the baseline phase was 71.1/100 prescriptions, which was alarming. Maximum errors were involved in writing prescriptions especially regarding dosage and interval. A UK-based review article on medication errors in neonates also reports incidence of medication errors, comprising 14%–74% of total error reports among 20 different articles with incorrect dosing being the most common error (42%).17
In our study, a considerable proportion of the errors were related to wrong interval or timing. In a South African study, it was also found that the most common problem was wrong interval of drug prescriptions, which was mostly associated with antibiotic prescriptions (284 of the 663 errors) like cloxacillin, cefuroxime, amikacin, imipenem, piperacillin–tazobactam and gentamicin,18 as the interval of certain antibiotics changes depending on the age of the neonate.
A good number of errors, as found by the researchers of the study, were transcription errors, which occurred while copying the medication orders to the monitoring sheets by the nurses. This may be attributed to illegibility of the prescriptions, heavy work load and repetitive desk jobs. This was corroborated by a similar study, based in Egypt, where total transcription errors were 227 out of 624 medication orders.19 Our study aimed to ease out this fallacy through computer-generated legible prescriptions, though specific methods for controlling transcription errors were not designed. Though the causal factors for high medication prescribing and administering error rates were not delved into, in the present research, still it can be ascertained that highly populated inpatient wards, inadequate staffing pattern, prolonged stressful shifts of the healthcare personnel, complex calculations, and change in dosage and intervals as per weight and age of the child are the major causative factors as represented in available literature.15 20
Quite a few research articles published abroad have documented various novel efforts to curb the rate of medication errors, though this domain however is scarcely studied in India. A multifaceted educational programme for the healthcare professionals was developed as a mode of intervention by the Patient Safety Committee of the Department of Paediatrics of a Buenos Aires hospital, which promoted a change in the approach to medication errors and on the development of a safety-oriented attitude for the sake of patient safety. The programme focused on education by means of different activities: grand rounds, interdisciplinary meetings, anonymous reporting of errors and other important policies. The result of which was reduction in the prescription error percentage from 11.4% to 7.3%, especially in the NICU where higher differences were observed (7.8%) (95% CI: 4.7% to 10.9%).21 Current endeavour too reflected some similar policies, like regular training of healthcare personnel, initially twice weekly in the intervention phase and then monthly in the post-intervention phase to bring forth improvement of the scenario. It was also ensured that new resident postings and rotation of nursing personnel were clubbed events following which special training sessions were conducted both by the QI facilitators and also the existing residents and nursing staff ensuring their active participation in the procedure.
A computer-generated order entry (CPOE) has been proven to be an effective intervention for preventing and reducing medication errors in prescriptions of paediatric and neonatal group in recent years which may or may not be provided with computerised decision support (CDS).22 In the course of our study, we designed software which was similar to this system of CPOE with CDS and it brought about remarkable improvement in prescription orders. Once a patient profile is created and the first prescription prepared, the subsequent prescriptions can be easily obtained by editing on the existing prescriptions of the same patient. Even the older prescriptions can also be retrieved from the memory of the database if required for evaluation. This web-based password-protected software is user-friendly and can be accessed through any digital platform.
Radley et al in their systematic review found that processing a prescription drug order through a CPOE system decreases the likelihood of error on that order by 48% (95% CI: 41% to 55%), corroborating with the results of our study.23
Though the different error indicators in our study were not significantly correlated with the gestational age of the neonates, other studies have commented prematurity to be a significant determinant for the occurrence of medication errors possibly due to longer periods of hospital stay or use of more drugs or complex calculations as causal factors.15 24
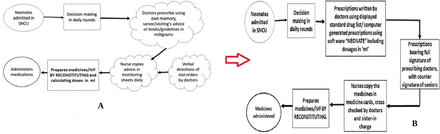
Sustainability of the implemented changes was a challenge throughout 12 weeks. Regular training continued imparting the information on rotating batches of nurses and residents. Use of the software over the internet was trained hands-on with the new incoming batch of residents and nurses. It was difficult to ensure full signature of the duty doctors, also cross-checking of all the prescriptions and countersigning of the senior members were not feasible throughout the 12 weeks. Use of software though, was popular and sustainable and was successful in maintaining low error rates. Constant contact was maintained with the software developers for any required changes in the system and also updating the drug list for any newly used drug in the set-up. A corrected process flow chart was now maintained in the set-up (figure 5B) correcting all the previously unclear and error prone steps (Fig 5A).
(A,B) Process flow chart in the baseline and post-intervention phase. IVF, intravenous fluid; SNCU, sick neonatal care unit.
Neonatal care institutions can undertake an anonymous survey to test the baseline medication error rates using some customised checklist. Using the QI model and team-based approach, training of the healthcare personnel could be started as the first change idea for improvement. This web-based software can then be easily used in any set-up, after pilot testing to generate prescriptions and minimise error rate.
The major changes were brought about by the prescriptions written in the morning shift. Healthcare personnel were eager to use the software in other shifts, to generate prescriptions. In the post-intervention phase, the prescriptions written in the evening shifts also had minimal or nil errors (data not in table). A change in attitude was also ushered in the nursing staff who were now much more confident in handling prescriptions and administering medicines.
This improvement was praised when it was discussed and displayed in time series charts in departmental meetings of the institution. Authorities planned to take up this task in paediatric wards and paediatric intensive care unit to study medication errors and use the QI methodology to deal with it.
Limitations
This project overall successfully implemented the POCQI methodology in dealing with the problem of medication errors. It offered an unbiased analysis of prescriptions, and proposed new ideas like developing and popularising new software for prescribing, with the outcome being sustained in subsequent periods. Limitations of the endeavour included primary focus on prescription errors for analysis and improvement targeted on that part only underestimating the drug administration errors. A sizeable proportion of the errors were in timing of the drugs administered, still there were problems in designing an appropriate PDSA cycle targeting the problem. Involvement of a full-time clinical pharmacologist in daily rounds and prescription scrutiny would have brought about further significant changes. As the prescriptions comprised mostly of critical patients admitted to the NICU, the duration of their hospital stay was longer resulting in low recruitment in each phase with respect to number of babies, though we could manage multiple prescriptions of them for analysis. Though no adverse drug reactions were noted, still categorisation of adverse drug reactions could have given another dimension for the study.
Conclusion
Using the QI model, which is at present an emerging saviour in various maternal and child health problems,25 this study could ultimately achieve its smart aim of restricting the medication errors to median medication error percentage of around 10%. The changes tested via PDSA cycles were also sustainable in the post-implementation phase, though there still remains a scope of betterment. The findings of this study generated interest among intradepartmental and interdepartmental sectors paving the way for further research in this topic. The software-generated prescriptions designed through this research can be a stepping stone to minimise medication errors in various healthcare settings and it thus brings us a new dawn of error-free prescriptions for the precious future generation of our country.
Data availability statement
Data are available upon request.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the Institutional Ethics Committee of the said institute (No/NMC/29), and proper informed consent was obtained from parents of each neonate whose prescription was enlisted and also the health personnel involved in the study.
Acknowledgments
We are thankful to Professor Tapan Kumar Sinha Mahapatra, Pediatrics, NRS Medical College and Hospital, Kolkata, for his valuable support and permission to carry forward the work in the department. We are thankful to all the healthcare personnel of the study setting linked directly and indirectly to the research.
References
Footnotes
Contributors SM—concept, design, literature search, data acquisition, data analysis, manuscript preparation, manuscript editing, guarantor. MB—concept, design, literature search, data acquisition, data analysis, manuscript editing. SM—literature search, data acquisition, manuscript preparation, manuscript editing. AM—concept, design, data analysis, manuscript editing. ND—design, literature search, data analysis, manuscript editing. BB—data analysis, manuscript preparation, manuscript editing. RG—design, data analysis, manuscript editing.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors. Publication of this article is made Open Access with funding from the Nationwide Quality of Care Network.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.