Article Text
Abstract
Background Kangaroo Mother Care (KMC) is a low-resource, evidence-based, high-impact intervention for low-birth weight (LBW) care. Quality improvement in KMC requires meso-level, macro-level and micro-level interventions. Our institution, a public teaching hospital, hosts a level-II/III neonatal intensive care unit (NICU). The average demand for beds typically exceeds available capacity, with 60% occupancy attributed to LBW patients. There was low uptake of KMC practice at our unit.
Aim statement In the initial phase, we aimed to improve the coverage of KMC in admitted eligible neonates from a baseline of 20%–80% within 15 days. After a period of complacency, we revised the aim statement with a target of improving the percentage of babies receiving 6-hour KMC from 30% to 80% in 12 weeks.
Methods We report this quasi-experimental time-series study. With the Point of Care Quality Improvement methodology, we performed Plan-Do-Study-Act (PDSA) cycles to improve KMC practice. We involved all the healthcare workers, mothers and caregivers to customise various KMC tools (KMC book format, KMC bag, mother’s gown) and minimise interruptions. Feedback from all levels guided our PDSA cycles.
Results The percentage of babies receiving at least 1-hour KMC increased from 20% to 100% within 15 days of August 2017. In the improvement phase, baseline 6-hour KMC coverage of 30% increased to 80% within 12 weeks (October–December 2017). It sustained for more than 2 years (January 2018 till February-2020) at 76.5%±2.49%.
Conclusions Quality improvement methods helped increase the coverage and percentage of babies receiving 6-hour KMC per day in our NICU. The duration specified KMC coverage should be adopted as the quality indicator of KMC. The training of healthcare workers and KMC provider should include hands-on sessions involving the mother and the baby. Maintaining data and providing suitable KMC tools are necessary elements for improving KMC. Minimising interruption is possible with family support and appropriate scheduling of activities. Having a designated KMC block helps in peer motivation.
- quality improvement
- PDSA
- teamwork
Data availability statement
Data are available upon reasonable request. Data is available with the corresponding author on request. We comply with BMJ’s policy of sufficient anonymisation to ensure that patients are not directly identified. The information is in the form of a master chart in MS Excel 2019 containing daily documented KMC hours received by each baby during her stay in the hospital over more than 26 months. It is available in four MS Excel sheets, having date-wise KMC hours in rows and individual babies as columns. Columns are colour-coded for a particular month.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known on this topic?
Kangaroo Mother Care (KMC) is a low-cost intervention known to reduce mortality in LBW babies.
What this study adds?
In LMICs sustaining improvement in KMC is feasible with system changes. The tools for KMC can be developed using QI approach. Family participatory care helps improve the quality of KMC in limited resources.
How this study might affect research, practice or policy?
This study may lead to further research into contextual system improvement to achieve sustainability in KMC rates.
Introduction
Prematurity is the most significant cause of neonatal mortality (35%) and long-term morbidities (3.1% of disability-adjusted life years) for all ages worldwide.1 2 Together, Asia and sub-Saharan Africa regions have the highest number (approximately 60%) of preterm births and low-birth weight (LBW) prevalence globally.3–5 In India, prematurity accounts for about 18% of live births.6 As per the National Family Health Survey-4, the burden of LBW is around 20% of live births.7
Kangaroo Mother Care (KMC) is a low-resource, evidence-based, high-impact intervention for LBW babies. WHO defines KMC as ‘early, continuous and prolonged skin-to-skin contact between the mother (or other caregivers) and the baby’.8 It is one of the global indicators of the quality of newborn care.9 10 Compared with conventional newborn care, KMC is known to reduce mortality by more than 50%.11 12 It reduces morbidities like severe infection by 42% and hypothermia by 23%.12 It also contributes to increased growth concerning weight, head circumference and length. It helps in breast feeding and bonding between the mother and the newborn.13 14
In India, the KMC movement started in 2003. Neonatologists from western and southern India led the training drive in Asian countries.15 16 The Government of India published guidelines for facility-based KMC to improve healthcare workers’ proficiency.13 Despite being an integral part of many guidelines and training modules, there is an implementation gap for KMC.16 17 The Government of India received KMC data from eighteen states in 2015–2017. The KMC coverage in Special Newborn Care Units was less than 20% in 12 out of those reported.18
The policy of KMC for LBW existed in our unit. We noted that the uptake of KMC was relatively low despite evidence of its benefits. This work aims to address the shortfall and the contextual issues for improving the process of care and experience of care.
The majority of quality improvement projects for mothers and newborns have looked at meso-level and macro-level interventions.19 20 Skill-based KMC training and reorientation improves the quality of care.21–24 In a quality improvement project, Joshi et al improved KMC with simple measures involving family members, positive reinforcement and felicitation of healthcare workers.25 26 In the initial phase, we aimed to improve the coverage of KMC in admitted eligible neonates from a baseline of 20%–80% within 15 days. After a period of complacency, we revised the aim statement with a target of improving the percentage of babies receiving KMC for at least 6 hours a day from 30% to 80% in 12 weeks.
Methods
This study was conducted in our institution, a public teaching hospital located in Maharashtra, India, with a capacity of 1100 beds. It caters to adjoining districts with a population of nearly 15 million people. Out of the approximately 18 000 births in a year, we admit about 3300 babies requiring intensive care in level II/III neonatal intensive care unit (NICU) with a capacity of 42 beds. A block in NICU comprises 12 beds with a nurse to patient ratio of 1:7. Our unit has a 24×7 visiting policy for either mother or female attendants. The average demand for beds typically exceeds available capacity, with 60% occupancy attributed to babies requiring KMC.
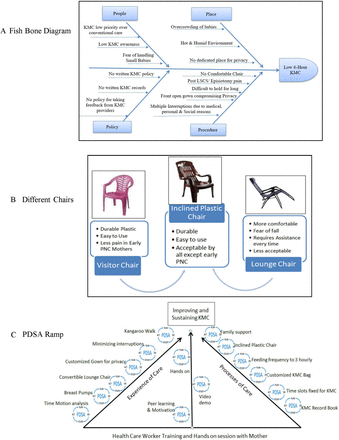
In the approach we adopted, we formed a team comprising the senior doctor (leader), in-charge nurse (communicator), nursing staff (recorder) and other members like mother, caregiver and housekeeping staff under the mentorship of an improvement advisor. We chose to improve KMC with a design of a quasi-experimental time-series study. We provided KMC to all admitted stable LBW babies weighing less than 2500 g. For this study, we considered stable LBW as eligible for KMC. We excluded all clinically unstable babies, those requiring oxygen and intensive phototherapy and whose mothers/caregivers were not available. Ishikawa fishbone analysis tool helped identify various root causes for low KMC coverage, as shown in figure 1A.
(A) Fish bone diagram for root cause analysis of low 6-hour-KMC. (B) Types of chairs and advantages for KMC. (C) PDSA pyramid showing: right ramp—processes of care which improved duration of KMC; left ramp—experience of care without impact on duration of KMC; base—provider skill and motivation through video demo, hands on sessions and continued peer learning necessary for improving and sustaining KMC. KMC, Kangaroo Mother Care; LSCS, lower segment caesarean section; PDSA, Plan-Do-Study-Act. PNC, post natal care mother.
Patient involvement statement
Mothers primarily provided KMC to eligible babies in this study. Another female family member replaced the mother if she was not available or exhausted. The KMC providers consented verbally at the time of admission. We actively involved mothers in the demonstration of the KMC position of the baby during training sessions. All the Plan-Do-Study-Acts (PDSAs) were patient-centric. KMC providers gave inputs for customising the KMC bag and selecting a comfortable chair. They also complied with reducing interruptions due to personal and social reasons. Confident KMC providers did the ‘kangaroo walk’ as and when required. Kangaroo walk is the term coined for ambulation while in KMC position to keep hands off the baby and instil confidence in KMC providers. We acquired the feedback from KMC providers also during informal discussions and Gemba walks. The team decided to adapt/adopt or abandon any idea after PDSA based on their feedback.
Measures
Indicator
We searched for the KMC quality indicators before developing and testing the changes. ‘KMC Coverage’ is defined as the percentage of LBW newborns receiving KMC. It is recommended and used across India as a quality indicator.13 24 26 In the initial phase, the team recorded daily KMC coverage of eligible babies for 15 days. This helped us to identify the limitation of the indicator. Any baby who received KMC for at least 1 hour on that day was a part of the numerator. It was easily achieved and did not explain the duration of KMC received by the baby. Hence, for the improvement phase, we revisited the indicator. The team decided on a different quality indicator for recording KMC. Some studies have taken the average duration of KMC in minutes or hours per baby per day.20–25 While it gives collective unit performance over the day, it falsely indicates that if the mean duration is 6 hours, all the babies have received KMC. Adding further details to the numerator would therefore give a clear understanding of improved care.9 Operational guidelines have defined ‘Extended KMC’ as 5–8 hours.13 Hence, we decided to record the ‘Duration specified (6 hours) KMC coverage’ indicator. We recorded the data for seven consecutive days and noted that it was feasible. Over the study duration, from 1 August 2017 to 29 February 2020, there were distinct phases of improvement, as shown in table 1. The indicators were different for the initial and improvement phases.
Summary of all phases describing various indicators
Interventions in initial phase
We tried different change ideas in this phase, as shown in table 2.
Summary of interventions in initial phase
PDSA for record-keeping of KMC: the team reviewed the status of KMC and emphasised the practice and documentation of KMC. The on-duty nurse started maintaining records on the individual case sheet in the daily morning shift. The nurse noted the previous day’s KMC hours and other information as reported by the mother. In a review meeting, the team noted excess non-relevant recorded data. The group decided to modify it into a KMC book format. The rows depicted individual babies; columns depicted KMC hours in each shift in the day. We revised it three times to make it easy so that data was available at a glance. We modified the number of rows in the format considering the average bed occupancy and the number of columns considering the average duration of stay.
PDSA for the training of KMC: during the Gemba walk, the team leader noticed gaps in the knowledge about KMC among staff on duty. The team discussed the knowledge gaps in a review meeting. The nurse in-charge suggested to conduct training sessions on KMC for the nursing staff. Hence, the team leader provided orientation to them concerning KMC and its advantages during the morning shift. These training sessions continued for 1 week. Though the KMC coverage increased, there was apprehension among mothers to keep tiny babies in KMC position. The trained team members conducted KMC audio-visual training followed by a hands-on demonstration on a willing mother-baby dyad. The team trained ten KMC providers (six mothers and four caregivers) and four nurses on that day. All KMC providers (except one who was unwilling) started practising KMC. The team members adopted this idea. The team continued to conduct the hands-on training sessions daily for 1 week. KMC coverage increased from a baseline of 20%–100% on day 15.
Phase of learning
Due to the early achievement of the aim set initially, team members thought the improvement is complete. The team felt that further action was not essential, and review meetings were not conducted during this period. The data recording stopped and returned to the prebaseline levels over 6 weeks. During a review meeting, our mentor analysed the situation and advised us to revisit the Point of Care Quality Improvement (POCQI) methodology.27
Interventions in improvement phase
After the review meeting with the mentor, we realised the gap and restarted work focusing on refinement in the initial phase interventions. The team documented the baseline data for 1 week. Tables 3 and 4 depict the summary of interventions in this phase.
Summary of interventions in improvement phase
Summary of interventions in improvement phase
PDSA for chairs: after the hands-on session, KMC providers, especially mothers and other female attendants (if the mother was not available or exhausted), gave feedback about the discomfort they experienced sitting on a visitor chair. Team members tried three chairs with different KMC providers, as shown in figure 1B. We inferred that each chair had a definite role and needed to keep all three in the unit. This helped to improve the duration of KMC further. We also experimented with a convertible lounge chair. This chair added comfort without an increase in the period of KMC. Acceptance was less in the beginning due to fear of fallback and assistance needed to operate it. The lounge chair got damaged in 2 months, as a result of which, we resorted to inclined plastic chairs for all KMC providers except for early postnatal mothers, who preferred visitor plastic chairs. The team also tried a bed with a backrest. It was comfortable for all KMC providers, but we had to abandon the idea due to the lack of space.
PDSA for tools to hold the baby in position: the team tried a local cloth binder and learnt that it was not comfortable for the mother and milk expression was impossible. Hence, we customised KMC bags to support the baby and help the mother express breast milk during KMC. We identified the need for three support stripes in the KMC bag during a trial on three Very Low Birth Weight babies. The tailor stitched the KMC bag with head, shoulder and back support as per the nurses’ suggestions and the mother’s feedback. We finalised two bag sizes, 1000 g–1500 g (small) and more than 1500 g (medium), depending on the crown-rump length.
PDSA for tools to provide privacy: the hospital supplied front open gowns for mothers. It was compromising their privacy during KMC. Hence, we modified it to include a flap in front as per the feedback from mothers and nurses.
PDSA to minimise interruptions: the team observed multiple reasons for interruption with time-motion analysis. Housekeeping, nursing procedures, clinical rounds and frequent milk expressions required 8 hours. Personal reasons like having meals, daily routine activities, meeting visitors consumed 5 hours. Night-time sleep and rest required 7 hours. Hence, only a cumulative 4 hours were available for KMC.
We allotted time slots for the housekeeping, NICU chores, mothers’ meals with visiting hours. The clustering of care minimised interruptions further. The manual expression of milk required half-hour (each time) before scheduled feeds. Hence, feeding frequency was changed from two hourly to three hourly, as it was more practical, though not a strong recommendation.28 29 We also performed PDSAs for the use of different breast pumps. They helped in milk expression without much impact on the duration of KMC.
The time required for diaper changes, mothers’ rest, night sleep and other daily living activities were beyond the team’s control. We encouraged the mothers to practice Kangaroo walk and provide KMC for as long as possible.30 The daily duration varied from 2 to 12 hours in a baby.
PDSA for the place of KMC: the team persuaded the hospital administration to designate the step-down section as ‘KMC Block’. It was located separately from NICU and was nearer to the postnatal ward. In the selected block, the team members could also provide focused education about KMC. Peer group learning was encouraged, and this helped the initiation of KMC for the new KMC provider.
Data collection and analysis
We considered the data on the first day of the initial phase as a baseline. On that day, the nurse on duty noted that three babies out of fifteen (20%) admitted LBW newborns received KMC. The team prepared a tabular format to record shift-wise KMC hours for all babies. The duration of KMC ranged from 0 to 12 hours per day in the same baby. After 15 days of the initial phase, the team noted significant lapses in recording data in the phase of complacency.
After restarting the quality improvement project, the on-duty nurse documented data for seven consecutive days following the phase of complacency. We noticed that 80% of babies received KMC, of which only 30% of babies received 6 hours a day. Hence, we changed our numerator to specify the duration of KMC. We decided this as the baseline for the improvement phase. We considered ‘KMC not given’ in that shift or day if the data were missing. The reason for incomplete data recording was a change in staff, lack of communication, and the record book frequently unavailable at the specified location.
We analysed the data using MS Excel 2019. We plotted the KMC coverage daily in the initial phase. Weekly average in the improvement phase, and monthly average in the sustenance phase. We used the mean and SD of the percentages for plotting on the statistical process control chart. The upper and lower control limits helped note and review common and special cause variations. We also documented the trending up of seven consecutive data points.
Results
Initial phase: the KMC coverage increased from a baseline of 20%–80% within 3 days after KMC record-keeping and healthcare worker sensitisation initiation. It rose to 95% in the next 2 days with an audio-visual session and hands-on with the mother. The first dip noted was a weekend with the senior staff on leave, as shown by annotation in figure 2A. The team achieved a KMC coverage of 100% within 2 weeks.
(A) KMC coverage (percentage of eligible babies receiving KMC for at least 1 hour) in initial phase at level II NICU in Government Medical College Hospital Aurangabad from 1 August 2017 to 15 August 2017. (B) Percentage of eligible babies receiving KMC for at least 6 hours in a day averaged and plotted against time on x-axis; weekly in improvement phase from 01 October 2017 to 31 December 2017 and plotted monthly in sustenance phase from 01 January 2018 to 28 February 2020 at level II NICU in Government Medical College Hospital Aurangabad; showing upper (UCL) and lower (LCL) control limits depicted in Levy Jennings chart with annotations. KMC, Kangaroo Mother Care; NICU, neonatal intensive care unit; PDSA, Plan-Do-Study-Act, HCW, health care worker.
Improvement phase
We reinstated the initial phase interventions (table 2) for KMC to note that the baseline 6-hour KMC coverage was 30%. It continued to rise steadily with the interventions depicted with annotations, as shown in figure 2B. During this phase, the dip was due to the festive vacation period. The replacement nursing staffs joining the duties were unaware of KMC.
Sustenance phase
We noted improvement in KMC by improving the experience of care and the processes of care as depicted in figure 1C. One of the significant milestones in the study was the designation of the ‘KMC block’ as a hospital policy. Documented KMC policy with allotted fixed time slots for interruptions became an operational norm. We displayed Information Education Communication material as posters in the KMC block and the waiting area. The dashboard at the entry of the unit indicated the KMC coverage of the day. Printed KMC book minimised the lapses of record maintenance. The data were reviewed monthly and plotted over a time series chart. The team noted that the average monthly percentage of 6-hour KMC from January 2018 till February 2020 was 76.5%±2.49% with control limits (upper 83.63% and lower 69.27%). There were no special cause variations as per Shewhart rules for the statistical process control chart.31 We could sustain the 6-hour KMC in 2018 (77.52%±2.3) and the year 2019 (75.9%±1) during the peak summer season, despite the hot and humid environment. There was no deviation from the mean or considerable dip in the consecutive vacation period from October to December 2018(77.23%±4) and 2019 (75.9%±0.8).
We noted sustained improvement in average 6-hour KMC coverage from January 2018 (74.4%) to February 2020 (76%). Based on the encouraging observations from this study, UNICEF offered financial aid, which resulted in establishing the KMC Centre of Excellence in October 2019. This has further played a vital role in the sustenance of KMC.
Discussion
Improvement in KMC coverage is an indicator of the quality of care. Bergh et al have proposed a monitoring model for institutional settings to quantify their progress in the implementation of KMC.32 The policy of providing KMC, interventions to implement it in routine practice, integrating the changes in the system, and sustenance are the different levels of progress in KMC. Initially, our team focused on increasing the percentage of KMC coverage of at least 1 hour per day for all eligible babies in routine practice.
KMC scale-up study group has given macrolevel and mesolevel interventions for sustainable KMC practice in India.33 Our study addresses microlevel interventions for integrating KMC into quality improvement.19 34 35 We learnt to set an appropriate goal for the team. Early achievement led to a phase of inactivity. We realised the gap during a review meeting with the quality improvement (QI) mentor at the end of 6 weeks’ passivity. The initial phase was a solitary person-centred effort. We learnt to shift from individual actions to team efforts for QI. We involved the team members in the subsequent improvement efforts. Improving KMC as any quality improvement initiative involves teamwork led by a champion.36 We had to face the challenge of frequent changes in the posting of KMC champions. Such changes are known to affect the unit’s KMC practices adversely, also noted in a study in rural western India.37
The team identified the need to redefine the context-specific KMC indicator.38 Joshi et al measured the mean duration of KMC as hours per baby per day.25 It suggests the overall performance of the unit in a day. We decided to record the ‘Duration specified (six hours) KMC coverage’ indicator, which was suitable in our context and easily replicable in other settings.
Though KMC is a low resource intervention, health systems have specific resource requirements.39 We learnt through POCQI to test interventions on a small scale for a short duration may optimise time and fiscal resources. Many change ideas failed or were abandoned at times due to inadequate resources. Many ideas involved various resources, most of which added to KMC provider comfort, which helped increase KMC hours. We could improve the experience of care as well as the process of care.
We studied interventions to improve KMC by enhancing the comfort for the mother and the baby through appropriate position. These tools helped the dyad to stay together for long hours. Of the interventions that we tried, inclined plastic chairs and customised KMC bags found ready acceptance. The customised KMC bag is the indigenous option available at a lower cost (INR 150) than the commercially available wrap.40
It is not possible to identify the most impactful interventions as they are interdependent and address different needs of the KMC process. All these interventions helped overcome the various resource-related and experiential maternal barriers.41 42 The KMC providers complied well with delivering KMC. There was no sociocultural barrier in contrast to the experience reported by Yue et al.43 It was observed that the fear of harming the baby, which is more at the initiation, subsides with support from nurses and other mothers. In the sustenance phase, continued peer motivation played a vital role in our project. Experienced mothers helped allay anxiety in new KMC providers, shared the benefits and various enablers for successful KMC. A study conducted in other low-income and middle-income countries has reported similar experiences.44
The commitment to implement KMC has come from all levels of hierarchy, including nurses, doctors, ancillary staff, mothers and the administration. This support and sustained teamwork fetched the centre of excellence recognition from UNICEF to our unit.
Generalizability
Similar health facilities elsewhere can extrapolate the ideas proposed in this study for maintaining records and providing better KMC tools. A low-cost inclined plastic chair (INR 1100) and a KMC bag (INR 150) offers a feasible and readily replicable option. The ability to minimise interruptions will depend on contextual factors. Lapses in records are a challenge in many public health facilities. Eliminating multiple entries and reducing data collection overhead by merging data into one register is feasible in all settings and would improve healthcare delivery and patient outcomes. Assigning a particular area and the time slots for KMC can help clustering of care and reduce turbulence of activities.
Strengths and limitations
The initial phase of our study focused on audio-visual and hands-on training for healthcare workers and the KMC providers, like some other studies.21–23 We demonstrated an increase in KMC coverage in a short span of 7 days, affirming the merit of learning by doing.
Another strength of our study is the context-specific KMC documentation format designed by our team. Such a structured layout is necessary for every dedicated KMC unit. Formal documentation and reporting emphasises the KMC policy implementation.
There was a period of inactivity following the initial phase, which led to a drop in achieved improvement in KMC. The team had to restart and reset the aim. A systematic review by Chan et al reported various components of KMC (kangaroo position, feeding, kangaroo discharge and follow-up).45 We looked at improving only one KMC component of the kangaroo position. There is no known dose-response effect of KMC.46 We need to continue our improvement journey to overcome specific challenges in our set-up and target ‘Continuous KMC’.13 The outcome measure for improvement in KMC should be optimal weight gain, reduced duration of the hospital stay, earlier time to achieve full enteral feeds, successful early discharge, and percentage of babies reaching 2500 g weight at 40 weeks postmenstrual age. We did not look at these outcome measures or any balancing measure like adverse events during KMC. Moreover, we did not include unstable neonates though shown feasible in sporadic studies.44 47 48 We did not extend the facility-based intervention to home-based KMC.
Conclusion
The findings from this study have shown that quality improvement methods helped increase the coverage and percentage of babies receiving at least 6-hour KMC per day in our NICU. The duration specified KMC coverage should be adopted as the quality indicator of KMC. The training of healthcare workers and KMC provider should include hands-on sessions involving the mother and the baby. Maintaining data and providing suitable KMC tools are necessary elements for improving KMC. Minimising interruption is possible with family support and appropriate scheduling of activities. Having a designated KMC block helps in peer motivation.
Data availability statement
Data are available upon reasonable request. Data is available with the corresponding author on request. We comply with BMJ’s policy of sufficient anonymisation to ensure that patients are not directly identified. The information is in the form of a master chart in MS Excel 2019 containing daily documented KMC hours received by each baby during her stay in the hospital over more than 26 months. It is available in four MS Excel sheets, having date-wise KMC hours in rows and individual babies as columns. Columns are colour-coded for a particular month.
Ethics statements
Patient consent for publication
Ethics approval
Institutional Ethical Committee approved the Quality improvement project No. IEC /419/2017, date 15 Sep 2017.
Acknowledgments
We are thankful to the administration, especially the Dean of our institute. We express our gratitude towards Dr Mahtab Singh, Dr Ankur Sooden, Dr Vikram Datta and NQOCN for the technical support. Mere thanks are not enough for our team of doctors and nurses who improved the overall quality of care with mothers of tiny babies willing to give KMC despite all odds.
References
Footnotes
Contributors All authors contributed and meet ICMJE criteria for authorship. AJ had full access to all of the study data and is guarantor for all the aspects of the work, including the decision to submit for publication. LD made substantial contributions to the study’s conceptualisation, interpreted the results, critically reviewed and revised the manuscript drafts, and approved the final manuscript as submitted. AL made substantial contributions to the study’s conceptualisation, provided additional data, interpreted the results, critically reviewed and revised the manuscript drafts. TJ designed the protocol, acquired data, interpreted the results, drafted and revised the initial manuscript, and approved the final manuscript as submitted.
Funding UNICEF funding only for logistics for KMC Centre of Excellence. No Award / Grant number. Publication of this article is made Open Access with funding from the Nationwide Quality of Care Network.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.