Article Text
Abstract
It is estimated that 1 in 10 hospital inpatients in Scotland have experienced a medication error. In our unit, an audit in 2019 identified documentation of as-required prescriptions on drug Kardexes as an important target for improvement. This project aimed to reduce the percentage of these errors to <5% in the ward in 6 months.
Weekly point prevalence surveys were used to measure medication error rates over a 12-week baseline period. Errors in route, frequency of dose and maximum dose accounted for >80% of all prescribing errors. The intervention was a poster reminder about the three most common errors linked to standards for prescribing pain medication. Barriers to change were identified through inductive thematic analysis of semistructured interviews with five ward doctors and two staff nurses.
In the 6 weeks after intervention, our run chart showed a shift in maximum dose errors per patient, which fell from 75% to 26%. However, route and frequency errors remained high at >70% per patient. Most of these errors were due to use of abbreviations, and qualitative interviews revealed that senior doctors and nurses believed that these abbreviations were safe. We found some evidence from national guidelines to support these beliefs.
Overall, the intervention was associated with decreased prevalence of patients without a maximum dose written on their prescription, but lack of space on drug prescriptions was identified as a key barrier to further improvement in both maximum dose and abbreviation errors.
- medication safety
- patient safety
- quality improvement
- hospital medicine
- qualitative research
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Problem
Inaccurate prescription documentation has the capacity to cause severe patient harm. A ward underwent audit of its patients’ Kardexes in mid-2019 to assess the scale of this problem, with feedback of results to the clinical team. Despite the existence of health board-wide prescribing guidelines in NHS Tayside,1 as-required or pro-re-nata (PRN) medications were found to be the poorest prescribed drugs, with accuracy between 20% and 40%. This was identified as an important target for improvement.
The ward is a 16-bed unit consisting of elective surgical patients, patients with cancer and emergency admissions with different prescribing demands. These patients often require pain medication and other as-required drugs such as antiemetics. The ward’s 3 monthly rotational intake of foundation year 1 (FY1) doctors was thought by the project team to have compounded the problem of inaccurate as-required prescribing as most prescribing in the ward is completed by doctors of this level. This project aimed to reduce the percentage of documentation errors of as-required medications in the ward within the 6-month intervention period.
Background
Most prescriptions in UK hospitals are written by doctors in their first two FY of training. Two large UK studies reported errors in 7.4%–8.4% of prescriptions written by FY doctors. Most patients in hospitals have more than one prescription, and consequently the prescription error rate per patient was 32%–50%.2 3 The most common suggested causes for these errors were in the work environment such as workload, interruptions, pressure from staff, cross-cover between wards and low staffing. Amongst individual factors, tiredness and stress were more frequent than insufficient knowledge or prescribing skills.2 Doctors have been found to adjust their performance to combat day-to-day challenges such as lack of time in the interest of patient safety; use of abbreviations would be an example of this.4 Areas of as-required prescribing that were commonly missed in studies on prescription documentation were ‘indication of drug’ and ‘frequency of dose’.5 6 These are examples of how doctors may skip what they deem as ‘less essential’ components of a prescription in the interest of saving time.
Prescribing is a four-step series of tasks: prescribing, transcribing, dispensing and administration.6 Each of these steps involves its own risks; however, evidence suggests that most errors occur in the ‘prescribing’ and ‘administration’ phases.6–8 A medication error is generally defined as an error occurring in any of these steps, whereas a prescription error occurs solely in the ‘prescribing’ phase.9 Prescribing can be further specified into regular medications (drugs the patient takes every day) and as-required medications (for symptomatic relief). Although as-required prescriptions are most commonly for pain, other common indications for this are nausea and vomiting, anxiety and allergies.10 A systematic review identified very little evidence about the safety or effectiveness of as-required prescriptions in comparison with regular prescriptions and suggested that PRN safety issues and adverse events are under-recognised.7 10 PRN medications require more written information than regular medications, creating more opportunities for error. The prescriber must include the indication for the drug, its frequency and its maximum dose in 24 hours. If these steps are not completed, the drug may be administered incorrectly, resulting in serious harm to the patient. Despite this, literature is scarce on how to improve PRN prescribing.11 12 Some work has been done into evaluating the efficacy of PRN prescribing in psychiatric units; however, there is limited evidence of audit into how well these medicines are prescribed for indications such as pain and nausea, which form the bulk of PRN prescriptions on our ward.13
Measurement
Quantitative methods
Initial data collection using a point prevalence survey (PPS) took place in the ward over a period of 8 weeks to quantify the number of errors in the as-required section of each patient’s Kardex. An average of seven Kardexes were assessed each week using a structured template (online supplemental material 1). Errors were classified using NHS Tayside prescribing guidelines. This guideline does not include a full list of accepted abbreviations; however, it does include strict guidelines on how to document the route of administration appropriately and rejects ‘PO’ as an appropriate abbreviation for ‘oral’ (online supplemental material 2). The guideline also specifically states that ‘Latin or other abbreviations must not be used’ and that the frequency of dose must be written in full. Furthermore, the guideline recommends that for as-required medicines, the prescriber ‘must state the indication and give a clear statement of dose, maximum frequency, and the maximum dose to be administered in each 24-hour period’.1
Supplemental material
The selected error domains based on these guidelines were no maximum dose included, improperly or omitted route of drug, improperly written or omitted indication of drug, improperly written or omitted frequency of dose, improperly written or omitted units of dose and ‘other’, which comprised any documentation error outside of the set domains.1
A primary outcome measure and a process measure were calculated from our PPS data:
Primary outcome measure: Percentage of patients on the ward with an as-required drug entry error.
Process measure: Number of as-required drug entry errors.
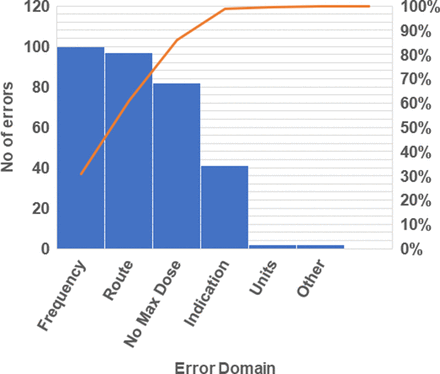
Of the Kardexes assessed at baseline for our primary outcome measure, a median of 50% of patients had an as-required prescription with an error. The most common errors were in the domains of route, frequency and maximum dose. The most common causes of these errors were omission of maximum dose or use of unsuitable abbreviations. The most common unsuitable abbreviations used were ‘PO’ instead of ‘oral’ when documenting drug route and a degree sign (°) written in place of ‘hourly’ when noting frequency. Pareto chart analysis of our process measure results in the preintervention period showed that over 80% of errors occurred in these three domains (figure 1).
Pareto chart of number of as-required drug entry errors by domain in the preintervention period.
We utilised the 80:20 rule when conducting our Pareto chart analysis, which states that about 80% of effects come from 20% of the causes.14 15 According to the chart, route, frequency and maximum dose errors were what contributed the most to the total number of PRN prescribing errors on the ward.
The intended outcome of the intervention was a reduction in adverse drug events, which was our intended process measure. This was emphasised in the poster statement ‘don’t put your patients at risk’ (online supplemental material 3). However, we could not measure adverse drug events for two reasons. First, they are uncommon; harm to patients occurs in <1% of undetected prescribing errors in hospitals.16 Consequently, large studies are required to detect the impact of medication safety interventions on patient outcomes.17 Second, the only available information about adverse drug events was the hospital’s system for reporting patient safety incidents. However, a study of 1006 admissions to an NHS hospital in England found that only 33 (11%) of 303 adverse events identified through case note review were reported on the hospital’s reporting system.18
We used qualitative methods to provide a balanced account of beliefs and attitudes about the intervention.19 We did not include quantitative balancing measures.
Qualitative methods
Qualitative semistructured interviews were conducted with five doctors and two nurses with informed consent. Interviews lasted approximately 20–30 min and were audio-recorded to allow for verbatim transcription. They took place in staff offices (n=3) and the ward’s family room (n=4) to ensure confidentiality for our participants. Interviewees were not provided with any information or sources about the topic prior to the interview.
Interviews used specific topic guides for doctors (online supplemental material 4) and nurses (online supplemental material 5) to ensure all key questions were covered while keeping the interview open to new ideas. The questions asked in the interview focused on confidence in writing (for prescribers) or reviewing (nurses) as-required prescriptions. This was because staff availability issues meant that some interviews (n=3) could not be undertaken in the postintervention period. As a result, our qualitative findings focused less on the effect of our intervention and more on staff opinions of PRN prescribing and its capacity for errors. Interview transcripts were uploaded to NVivo V.12, and data management, coding and initial analysis were conducted using this software. Inductive approaches were used throughout the thematic analysis of the transcript to allow the interviewees’ themes to arise from their own discussions.
Design
A small quality improvement (QI) team consisting of a medical student (SR) and a consultant (YB) was formed. Patients were not involved in the design, conduct, reporting or dissemination plans of our research as this was not feasible given the time frame for the project. Environmental factors for inaccurate PRN prescribing were identified through creation of a cause-and-effect diagram (see online supplemental material 6) using findings from two ward observation sessions conducted by the project lead (SR). These were applied to the COM-B Model of Behaviour to identify target barrier behaviours for a behavioural diagnosis.20 From our analysis of the model (online supplemental material 7), ‘opportunity’ was found to be the dominant COM-B component in which barrier behaviours to accurate PRN prescribing were driven, with ‘physical opportunity’ in the context of the ward environment emerging as the dominant barrier behaviour. This information was then applied to Michie’s Behaviour Change Wheel.20 We identified three potential intervention functions—persuasion, incentivisation and coercion—through the means of either environmental or social planning, communication, marketing or legislation. The busy nature of the ward meant that junior doctors required a quick and effective tool to help them with PRN prescriptions. A poster intervention was selected as the team felt it was a good combination of each intervention function and was manageable given the time frame for the project. Most prescribing took place in the ward’s doctors’ room; therefore, a poster in this location was most suitable for our target population to maximise ease of access.
Our poster was developed using NHS Tayside’s prescribing ‘pain ladder’ guideline, which contains our health board’s recommended pain medication for patients across all specialties who have mild, moderate or severe pain.21 The poster also focused on prevention of errors in the three main error domains identified from our baseline data collection (online supplemental material 3). The poster was laminated and put up in the doctors’ room as well as other key areas where prescribing tasks were known to take place.
Data collection was reinitiated after the introduction of the poster and was conducted in the same fashion as the preintervention data to assess for improvement in as-required prescribing. Plan-do-study-act (PDSA) cycles were used to monitor the intervention and provide modifications where appropriate (online supplemental material 8).
Strategy
Our SMART aim was to decrease as-required documentation errors on drug Kardexes to <5% by April 2020. We used a set of PDSA cycles to monitor and adapt our intervention design to ensure our method was suitable for the ward environment.
Our data collection method was a weekly PPS using a template (online supplemental material 1) to gain a ‘snapshot’ of patient Kardexes at a set time each week. It was predicted that collecting data in this way would give the team an understanding of the rate at which PRN prescribing errors occurred on the ward.
PDSA cycles
A poster was introduced to the ward to remind doctors of best practice when prescribing as-required medications. The project team wanted to maximise the potential of the intervention through PDSA cycles. These represent planning (PDSA 1.1) and design (PDSA 1.2) of the poster and redevelopment of the poster using cues from the internal environment and staff behaviours (PDSA 1.3).
PDSA cycle 1.1 (October 2020): The team planned to use up-to-date pain guidelines to form the content of our poster. We searched various guidelines to create the template; however, no updated material was available. The team thought this was because the content was being accessed via a personal computer instead of an NHS computer. We accessed an NHS computer with Staffnet to print out the most recent pain ladder guidelines for Tayside; this was then used as a template to create a pain ladder poster.21
PDSA cycle 1.2 (November 2020): An A4 poster was created and put up on the doctors’ room noticeboard. Ward prescribers and reviewers were informed about the poster and its purpose to ensure staff knew where to seek help if they needed it. One week after the poster was introduced to the noticeboard, it had been moved by a member of staff behind other guidelines on the wall. A new location was sought to prevent staff tampering with the poster.
PDSA cycle 1.3 (November–December 2020): The poster was laminated to increase its durability and had bolder colours to attract the eye of FY1s working at their station. More posters were printed out and added to other areas of the ward where prescribing was known to take place, such as the dispensing room and the nurses’ tables at the end of each bed bay. Staff were informed of the new locations of the posters. One week later, there was no further movement of the posters. These locations triggered a slight improvement in PRN prescribing as after intervention there was a 39.2% decrease in the number of prescribing errors on patients’ Kardexes.
Additional details for each PDSA cycle are in online supplemental material 8.
Results
Qualitative results
From the quantitative baseline data, the two most common error domains were that of ‘frequency’ and ‘route’ because of the use of abbreviations (figure 1). The most common term used to abbreviate the frequency of dose was a degree (°) sign in place of writing out ‘hourly’ in full, and the most common abbreviation found in the ‘route’ domain of the Kardex was ‘PO’ in place of writing out ‘oral’. These were the most common prescribing errors seen throughout the entire data collection process. Staff opinions were investigated using semistructured qualitative interviews with ward staff to discover whether they thought using abbreviations on drug Kardexes was appropriate.
In summary, interviewees used abbreviations in the Kardex for three main reasons: common knowledge, habit and because of physical barriers such as lack of space to write a full prescription. Although physical barriers and habit were justifiable reasons for abbreviating prescriptions from the data, there were discrepancies between the doctors’ and nurses’ opinions on how easily their prescriptions were interpreted by nursing staff, with illegibility being a key issue for Staff Nurse A. Interview data provided significant evidence that there was an abbreviations-supportive culture on the ward resulting from these contributing factors. This provided the team with insight as to why the quantitative errors detected were most often from abbreviations of drug route and frequency. Qualitative interviews also provided us with information on why maximum dose was often missed out by prescribers; this was because it involves a lot of writing in a small space. Hierarchical themes from the interviews are summarised in online supplemental material 9.
Quantitative results
Results for our outcome measure data preintervention and postintervention were expressed in the form of run charts for each error domain: route, frequency and maximum dose. Results were collated into preintervention weeks (1–7) and postintervention weeks (1–8). Data for preintervention week 1 were not formally recorded as pilot data collection took place at this time. Data for maximum dose errors were not recorded until preintervention week 4 because this information was not originally included as one of the error domains in our template. A ‘maximum dose’ error domain was added after the team found poor compliance with maximum dose documentation in the first 3 weeks of data collection (online supplemental material 1).
Run charts showed that the intervention was not associated with any change in the frequency of errors in the domains of route (figure 2A) or frequency (figure 2B). The run chart for maximum dose errors (figure 2C) did show some improvement after the poster was introduced. A shift (circled) can be seen on the run chart from weeks 3 to 8 after intervention, with 7 points below the median. This indicates there was a non-random change to the prescribing process during this time. No trends or astronomical data points were present. Error rates over both the preintervention and postintervention period are summarised in online supplemental material 10.
Run charts for the three most common as-required prescription errors preintervention and postintervention.
Lessons and limitations
The main lesson learnt from this project was that prescribing is a multifaceted task that is the responsibility of not only the prescriber but also reviewers such as nursing staff and pharmacists. Kardexes were reviewed by nurses two times per day and less frequently by pharmacists. At the time of data collection, there was no set ward pharmacist. Lack of support during the review process may have contributed to why errors were not being picked up or communicated to doctors effectively at the time.
Abbreviations were the most common error and were unaffected by our poster intervention. Lessons were learnt from the interviews about likely causes. Both consultants said they would use abbreviations regularly (table 1). In contrast, two of the three junior doctors interviewed showed awareness that they were not supposed to abbreviate prescriptions. Importantly, both nurses were content with doctors using abbreviations if handwriting was legible. A previous qualitative study with 22 FY doctors found that senior doctors were seen to be highly influential to their prescribing behaviour through both informational (the influence of other people’s knowledge and skills on our behaviour) and normative (the influence of what other people expect one to do) pathways. These interviews also revealed a common belief that prescribing errors are not likely to have consequences for patients.22 This led us to reflect on the evidence about consequences of the abbreviations that were common in our ward. We found that the abbreviation ‘PO’ does not appear in the US Joint Commission’s official ‘Do Not Use’ list of abbreviations23 and is specifically included as safe in the Australian Commission on Safety and Quality in Healthcare’s national recommendations.24 The (°) abbreviation is not in the US ‘Do Not Use’ list, but the Australian recommendations say, ‘Do not use symbols. Avoid, for example, “°2” to mean “every 2 hours.”’ However, it is not in their list of high-risk abbreviations.24 An example of a high-risk abbreviation is ‘U’ for units. For example, prescription of 6iu (6 international units) has been interpreted as 61 units and prescription of 10U (10 units) has been interpreted as 100 units. In both cases, the patients were harmed; the first was admitted to hospital and the second transferred to intensive care.25 The lesson that we have learnt is that the abbreviations we identified are not high risk. Nonetheless, nursing staff find doctors’ handwriting hard to read, and abbreviations exacerbate this problem (table 1). The policy on abbreviations applies to all hospital units in NHS Tayside, and consequently changing the policy on a single ward is not an option.1
Doctors’ and nurses’ attitudes to abbreviating prescriptions on a drug Kardex
None of the interviewees questioned the need to specify a maximum dose, yet our poster intervention was associated with a reduction in maximum dose errors (figure 2C). However, reduction in these errors began before the intervention, which may have been due to a Hawthorne effect; the need to prescribe maximum doses was discussed with staff in the first 3 weeks of data collection when its documentation in the Kardex was found to be infrequent.26 Despite improvement, the postintervention median for maximum dose errors was 22% (online supplemental material 10), which is well above the target of <5%. Interviewees identified lack of space on the Kardex as a major barrier to specifying maximum doses and eliminating abbreviations.
Only one person (SR) was responsible for all quantitative and qualitative data collection and analysis, which may have introduced unintentional bias to the project. The investigator visited the ward every week and therefore got to know ward staff, of whom some were also participants in qualitative interviews. Interviewer bias was avoided, where possible; however, participants could have felt influenced to answer questions in a certain way because of the investigator’s role as a medical student.
The main limitation in this study lies in its generalisability, as the project took place in a single hospital ward. As the sample size was low in terms of ward capacity (n=16), the findings from this study are unlikely to be generalisable to other areas. This weakens our project’s sustainability as it renders us unable to benefit from spreading our project beyond our local area through word-of-mouth (diffusion) or poster redistribution (dissemination); these are key elements of a sustainable QI project according to the diffusion and dissemination paradigm.27 28 As the ward contains a high volume of surgical patients, many Kardexes were with the patient in surgery at the time of data collection or were missing. This meant the sample size of Kardexes was low, with an average of seven available each week for audit.
Furthermore, other issues surrounding sustainability are important to consider as FY1s rotate every 4 months to a new area; the new cohorts would therefore have to be informed of the poster for it to be fully effective. As the lead researcher (SR) was a medical student based on the ward for 12 months, this was only possible for those who joined the ward during this period. Other team members were either not ward-based (PD and SG) or were consultants (YB) who are only on the ward for a short space of time. This demonstrates how involvement from staff members working in the target environment is essential for the sustainability of an improvement project.
Our results show that a poster intervention alone is not sustainable or sufficient to improve all elements of prescribing on the ward. Around 70% of planned organisational change fails, and this is most often a result of design flaws in the planning stage of a project.27 NHS Improvement divides this into three areas: staff, organisation and process.29 More effort should have been made to involve our target group in the design of our project; this could have been done by conducting interviews earlier in the year during our preintervention stage. Organisational factors such as availability of prescribing tools and staff training could have been evaluated in more detail. Furthermore, the process-related benefits to the system could also have been evaluated, for example, does accurate prescribing make other parts of the system run more smoothly? More communication regarding the benefits of accurate PRN prescribing should have been made clear to patients, staff and the organisation to maximise sustainability.29
Our intervention was a simple, cost-effective method of eliciting improvement and was easy to distribute throughout the ward. However, this is a passive method of changing practitioner behaviour and did not bring PRN error rates down to our target of <5% by 6 months. A poster can also be easily moved or misplaced, which further impedes its efficacy, as PDSA cycle 1.2 demonstrated. The use of passive interventions such as posters to prevent erroneous behaviours has been successful30 31; however, evidence shows it is more effective when done in combination with an active intervention.32 33 Qualitative interviews with ward staff were a strength of our project; by examining the contrasts and similarities between doctors and nurses on the ward, we had the opportunity to understand the views of our stakeholders. Solutions were also offered by interviewees such as providing more space to write in the Kardex. A key recommendation from this project is to involve the target population early to implement their views into the project design.
Conclusion
This study aimed to improve documentation accuracy of as-required prescriptions on hospital inpatients’ drug Kardexes. A poster intervention introduced to the ward was successful in reducing the ward’s rates of maximum dose documentation errors but had no impact on abbreviations. Our work shows that passive dissemination of posters has some capacity to enact change in prescribing behaviour; however, we acknowledge that further progress with reducing prescribing errors on our ward is likely to require redesign of the drug Kardex to allow for more space to write prescriptions in full. The next steps for this project involve taking on board the suggestions from our qualitative interviews to redesign a new Kardex for the ward to facilitate PRN prescribing.
Improving PRN prescribing involves a multisystem effort from doctors and nurses to encourage safe practice; however, outside pressures continue to impede this process.34 35 A supportive approach to safety is required in healthcare to provide prescribers with the opportunity to learn from their errors to both inform their own and others’ learning.4 In addition to this, review of local guidance for abbreviations on prescriptions should be encouraged across all health boards
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Acknowledgments
The lead author would like to thank Dr Suzanne Grant, Professor Peter Davey and Dr Yeshi Bhushan for their continued support and enthusiasm for this project. Their ideas and determination for improvement in prescribing were what allowed this improvement project to come to fruition.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors SR was the lead researcher for the project and was responsible for the overall content of this paper. SR conducted all data analysis and interpretation. YB had the original idea for the project and was also involved in recruitment of qualitative interview participants for the project. PD and SG were responsible for designing the methodology of this project. PD, YB and SG provided feedback and comments regarding the content of this paper. SR submitted the paper.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.