Article Text
Abstract
Background Often the first opportunity for clinicians to assess risk of preterm birth is when women present with threatened preterm labour symptoms (such as period-like pain, tightening’s or back ache). However, threatened preterm labour symptoms are not a strong predictor of imminent birth. Clinicians are then faced with a complex clinical dilemma, the need to ameliorate the consequences of preterm birth requires consideration with the side-effects and costs. The QUiPP app is a validated app which can aid clinicians when they triage a women who is in threatened preterm labour.
Aim Our aim was to produce a toolkit to promote a best practice pathway for women who arrive in threatened preterm labour.
Methods We worked with two hospitals in South London. This included the aid of a toolkit midwife at each hospital. We also undertook stakeholder focus groups and worked with two Maternity Voice Partnership groups to ensure a diverse range of voices was heard in the toolkit development. While we aimed to produce the toolkit in September 2020, we rapidly rolled out and produced the first version of the toolkit in April 2020 due to COVID-19. As the QUiPP app can reduce admissions and hospital transfers, there was a need to enable all hospitals in England to have access to the toolkit as soon as possible.
Results While the rapid rollout of The QUiPP App Toolkit due to COVID-19 was not planned, it has demonstrated that toolkits to improve clinical practice can be produced promptly. Through actively welcoming continued feedback meant the initial version of the toolkit could be continually and iteratively refined. The toolkit has been recommended nationally, with National Health Service England recommending the app and toolkit in their COVID-19 update to the Saving Babies Lives Care Bundle and in the British Association of Perinatal Medicine Antenatal Optimisation Toolkit.
- women's health
- obstetrics and gynaecology
- maternal health services
Data availability statement
All data relevant to the study are included in the article.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background and the problem
In England and Wales, approximately 8% of babies are born preterm (before 37 completed weeks of pregnancy). The leading cause for deaths under 5 years of age is prematurity, and those that do survive have high rates of long-term sequalae.1 2 The economic effects of preterm birth in England and Wales have been estimated at £2.95 billion per annum.3
Often the first opportunity for clinicians to assess risk of preterm birth is when women present with threatened preterm labour symptoms (such as period-like pain, tightening’s or back ache). Threatened preterm labour symptoms are not a strong predictor of imminent birth, as only 3%–5% of women who present with symptoms will deliver within 7 days.4–6 Clinicians are then faced with a complex clinical dilemma, the need to ameliorate the consequences of preterm birth requires consideration with the side-effects and costs. Clinician’s often ‘err on the side of caution’ when triaging women in threatened preterm labour,7 meaning most women receive unnecessary treatments and interventions. These interventions include antenatal corticosteroids, admission to hospital or an in-utero ambulance transfer to another hospital. This complex clinical dilemma is exacerbated by current guidelines which advise a treat-all policy for women presenting in threatened preterm labour before 30 weeks.8 9
To aid accurate prediction of preterm birth, the QUiPP app was developed by our group at King's College London and Guy's and St Thomas' NHS Foundation Trust.10 11 It is free to download through iOS, Android and also available through a website version at: www.quipp.org.
The app is able to predict the woman’s risk of delivery within clinically important timeframes (within 1 week, 2 weeks, 4 weeks and before 30 weeks’, 34 weeks’ and 37 weeks’ gestation) through combining their medical history (previous pregnancy information and risk factors for preterm birth), current pregnancy information (gestation, whether they are carrying singleton or twins) and predictive clinical tests (a cervical length measurement by transvaginal ultrasound and/or a swab result called quantitative fetal fibronectin). The QUiPP app has been validated and recently been updated to Version 2 (based on 1032 women with symptoms of premature birth) and demonstrates excellent predictive ability.11 QUiPP Version 2 has also achieved a CE Marking as a Class 1 Medical Device (MHRA Ref. no. for Medical Device/standalone software Z301 registration is A015030).
Organisational context
Analysis from our group demonstrated that using the QUiPP app when triaging women who arrive in threatened preterm labour could reduce hospital admissions and associated costs by 89%.12 Through working on the EQUIPTT (Evaluation of the QUiPP App for Triage and Transfer) Study (REC ref: 17/LO/1802)13 (which sought to evaluate whether QUiPP enabled clinicians to make more appropriate management decisions when women arrived in threatened preterm labour), our trial team gained experience on implementing the QUiPP app in different organisational contexts. Qualitative interviews with women and clinicians during QUIPP app use in this trial highlighted that clinicians were supportive of the app and found it useful when triaging patients. However, the interviews also revealed challenges in adopting new technologies and disseminating change of practice. These challenges included the need to emphasise that the app is a clinical decision support tool (rather than a diagnostic tool) and misunderstanding some of the app fields (as not aware of the ‘information’ section in the app).
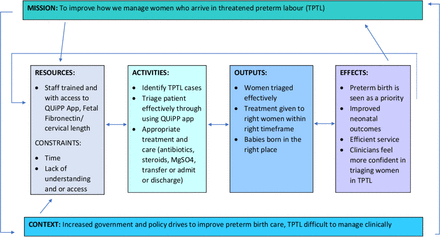
One approach to bridging this implementation gap14 is to create a toolkit of resources.15 Toolkits have been used for decades to enable clinicians to implement evidenced-based practice.16 In UK preterm birth maternity care, the use of toolkits was established through the hugely successful PReCePT Toolkit.17 A toolkit develops practice pathways and a range of tools (such as presentations for mandatory training, audit tools, evidence summaries, patient information leaflets and frequently asked questions documents) to ensure the project can be easily implemented and embedded in different hospital units, achieving high satisfaction.15 An initial needs assessment was undertaken,18 which was later refined (see figure 1).
Logic model for The QUiPP App Toolkit.18
Implementing the solution
To facilitate us in aiding implementation of the QUiPP app across other hospital units, we successfully applied for funding to develop The QUiPP App Toolkit.
Our aim was to produce a toolkit to promote a best practice pathway for women who arrive in threatened preterm labour. This 12-month project begun in September 2019, with the aim of producing a completed toolkit by September 2020.
We worked closely with two busy hospitals in South London, who serve a diverse local community and who had both been involved in the EQUIPTT Study.
It has been suggested that toolkit-like strategies should be developed after intense learning has been undertaken from a small number of hospitals or sites.19 These two hospitals were therefore chosen because during the EQUIPTT Study, one of the hospitals had implemented the QUiPP app well, and one had not, enabling us to uncover what worked well, and what did not work well.20 A member of our research team (HAW) was also returning to clinical work at one of these units, giving us the opportunity to have an ‘insider researcher’ embedded in the local delivery of maternity care.21 22 With the awarded funding we paid for a toolkit midwife at each hospital (10% whole time equivalent, for 3 months each) to act as a ‘champion’.23 24 This allowed us to have staff who were able to dedicate time to the QUiPP Toolkit rather than trying to fit it around their busy clinical schedules, working alongside The QUiPP Toolkit Group (NC, HAW and AHS). The role of the two toolkit midwives was not to implement the toolkit at their unit, but to communicate their views and individual site knowledge on what aided or hinder implementation of the QUiPP app during the EQUIPTT Study, and what could aid implementation of the app and the toolkit moving forward. Some of this knowledge (for example around staff culture or hierarchies) would have been difficult to deduce as external staff.24
The combined experiences of implementing the QUiPP app as part of the EQUIPTT Study, our own clinical experiences, discussions with the toolkit midwives at both sites, and telephone support from the experienced PReCePT Toolkit team, refined our logic model18 (figure 1).
We were keen for engaged participation from stakeholders in developing the toolkit,25 ensuring we garnered a range of views and feedback from a variety of people. We held two stakeholder involvement events, one at each hospital. Both stakeholder events were held in January 2020, on a weekday evening lasting 2 hours. The scheduled date was determined via consensus of invited participants (face-to-face or via an electronic calendar availability poll). This gave us enough time for stakeholders to contribute their perspectives, but were pragmatic and flexible to fit around their busy schedules.26 The events were advertised via word of mouth and email through the toolkit clinicians at each site. At the stakeholder involvement events refreshments were provided, alongside shopping vouchers to thank attendees for their time. At our first event, there were 19 attendees, and at our second event there were seven attendees. The attendees at both events included representation from admin staff, healthcare assistants, maternity support workers, midwives and obstetricians.
Both stakeholder events followed the same format. First, we went through the logic model and explained what we wanted to achieve with the toolkit with their engagement.27 We then spent time going through a hypothetical symptomatic patient’s journey (from attending the hospital to being either discharged or admitted) and considering how could pathway be improved, and what would help achieve this. Finally we went through the ‘Will It Work Here?’ questionnaire guide28 to expose any factors that needed considering to ensure that the toolkit would support a pathway to ensure best practice.
While NC and HAW chaired the sessions to ensure we stuck to time, the events had a relaxed atmosphere, with attendees urged to contribute throughout and encouraged to talk freely. To aid free conversation, the events were not recorded. However, NC did scribe important points on large whiteboard paper, so attendees were able to view exactly what was being logged.
At the stakeholder events, we jointly decided on what tools would be useful to include, and feedback from the two groups highlighted what inclusions would be valuable (figure 2).
Tools to include in toolkit from stakeholder events. FAQ, Frequently Asked Questions; fFN, Fetal Fibronectin.
Using the QUiPP app requires the clinician to input the result of a clinical predictive test (quantitative fetal fibronectin and/or cervical length measurement). Quantitative fetal fibronectin is more commonly used in the triage setting as undertaking cervical length measurement requires additional scan training. With the support of our funder, Health Innovation Network South London, we made plans to collaborate educationally with innovative medical technology company, Hologic (the only UK supplier of quantitative fetal fibronectin). This enabled us to include information in the toolkit that is vital to the smooth running of a threatened preterm labour pathway (such as quantitative fetal fibronectin ordering, calibration and quality control checks). The collaboration also aided dissemination, first through hosting a free QUiPP Toolkit webinar for clinicians across Europe, and second through giving the opportunity for the QUiPP team to train the quantitative fetal fibronectin UK representatives so they felt confident discussing and disseminating QUiPP with local sites. While the active educational collaboration with Hologic ensured appropriate technical and dissemination support, there is no financial benefit to the involved parties. Intellectual property of the toolkit solely remains with The QUiPP App Toolkit Group (NC, HAW and AHS).
In late February 2020 (a month after the two stakeholder events were held), we presented a first draft of one of the tools (the ‘QUiPP evidence quick sheet’) at a continual professional development event for maternity staff in South London. This was to gather feedback, to allow continual refining of the tools. The audience of midwives and obstetricians at this evening teaching session (the majority of whom were not at our previous stakeholder events) all approved of the idea of the toolkit, and our draft. They did not advise any modification to the ‘QUiPP evidence quick sheet’ tool we presented. We planned future steps which included producing individual components of the toolkit to stakeholders, allowing us to gather feedback from staff and women on each component to ensure the development remained an iterative process.
Three and a half weeks later, the UK Government announced that the UK would be in lockdown due to COVID-19. Immediately concerns were raised that the pandemic would increase strain on hospital beds and ambulances.29 As the QUiPP app reduces inappropriate hospital admissions and inappropriate ambulance transfers,12 interest in The QUiPP App Toolkit intensified at a variety of hospital sites.
The West of England Academic Health Science Network contacted us and asked if we would be able to rapidly roll out The QUiPP App Toolkit so they could use it within their region. Within 2 weeks we produced a Version 1 of the toolkit based on feedback from our two stakeholder events. We contacted two local Maternity Voice Partnership groups whose members reviewed our patient facing leaflets. Through adaptable and flexible working they were kindly able to provide us with comments via email.26 The British Association of Perinatal Medicine kindly agreed to host the toolkit on their website. We pragmatically followedthe toolkit development recommendations as best we could in the pandemic circumstances.30 On 7 April 2020, Version 1 of The QUiPP App Toolkit was rapidly rolled out and available for free (www.bapm.org/quipp).
The launch of The QUiPP App Toolkit was publicised via social media accounts including those of the QUiPP app, Health Innovation Network South London, the British Association of Perinatal Medicine and the PReCePT team. NC also emailed the 44 Local Maternity Systems in England to make them aware of this free resource. Alongside the toolkit being included as part of the West and South West of England PERIPrem care bundle,31 the Pan-London Maternity Clinical Network has also recommended the app and toolkit to their network. There has also been national recommendation, with National Health Service (NHS) England recommending the app and toolkit in their COVID-19 update to the Saving Babies Lives Care Bundle32 and in the British Association of Perinatal Medicine Antenatal Optimisation Toolkit.33
Since the toolkit has gone live, we have had numerous emails with sites across England to help them embed the toolkit. We have also delivered several free online training sessions to aid toolkit implementation in hospital sites country wide, from Lancashire to Surrey.
Unresolved questions
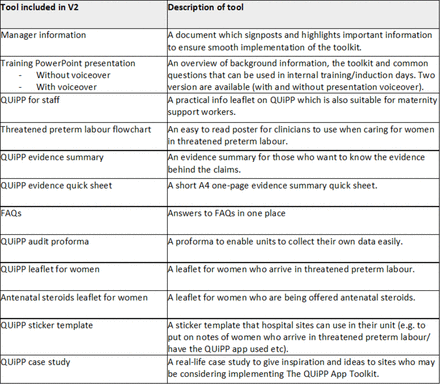
Rapidly rolling out The QUiPP App Toolkit has enabled hospital sites to have earlier access to resources which help facilitate a best practice pathway and use of the QUiPP app. However, the rapid rollout meant the original iterative process of developing the toolkit was modified. While Version 1 of the toolkit was available, we remained open to continual feedback to ensure this iterative process was not lost and the toolkit was continually refined. Feedback gained since Version 1 went live has comprised of including a leaflet on antenatal corticosteroids, QUiPP risk stickers, case site examples, a video (which was discussed pre COVID-19 but unable to be executed during lockdown) and a voice-over on the PowerPoint presentation (so it could be uploaded for mandatory online training). All feedback has been incorporated into Version 2 of the toolkit (www.bapm.org/quipp). A full list of tools included in The QUiPP App Toolkit can be seen in figure 3.
Tools included in The QUiPP App Toolkit. FAQ, Frequently Asked Questions.
Women’s voices were heard in the development of Version 1 through the feedback from Maternity Voice Partnership. While this enabled us to have some patient and public involvement while working within the constraints of a COVID-19 lockdown, it is still an area that we are aiming to enhance for Version 2. This includes trying to ensure we have feedback from women who fully represent the diverse group who present in threatened preterm labour. Given ongoing social distancing measures, future patient input may need to be virtual. While there are disadvantages to virtual meetings (such as the difficulty in recognising non-verbal cues, internet and technical issues, and the impact these can have on group dynamics), as with telephone interviews,34 the increased accessibility and convenience may well enhance our efforts to reach a more representative group of women.
Lessons for the field
While the rapid rollout of The QUiPP App Toolkit due to COVID-19 was not planned, it has demonstrated that toolkits to improve clinical practice can be produced promptly. Through actively welcoming continued feedback meant the initial version of the toolkit could be continually and iteratively refined, producing a result which may be superior to our pre COVID-19 vision. It is likely that this project may be one that has benefitted from the appetite for change and new ways of working which have accompanied the COVID-19 pandemic’s effect on the NHS.
We are pleased to produce a toolkit (www.bapm.org/quipp) that is recommended nationally,32 and enables hospital sites to improve their care pathway for women who arrive in threatened preterm labour. Through working with the expertise of Health Innovation Network South London, our future aim is to increase spread and adoption of The QUiPP App Toolkit to achieve full coverage throughout England.
Data availability statement
All data relevant to the study are included in the article.
Acknowledgments
The development of the QUiPP App was funded by the Guy’s and St Thomas’ Charity (Registered Charity No. 1160316) and Tommy’s (1060508). The development of the QUiPP App toolkit was funded by a Health Innovation Network South London ‘Innovation Award 2019/2020’ awarded to: Ms Naomi Carlisle, Dr Ellie Watson and Professor Andrew Shennan. We would like to give acknowledgements to: Health Innovation Network South London, British Association of Perinatal Medicine, Lewisham and Greenwich NHS Trust, Ms Sara Coughlin (Preterm Birth Champion Midwife at Queen Elizabeth Hospital, Woolwich), Ms Henretta Coker (Preterm Birth Champion Midwife, University Hospital Lewisham), Dr Maria Pinto Correia (Obstetrician at University Hospital Lewisham who kindly produced the Antenatal steroids leaflet for women), Ms Emma Wayman (Senior Research Midwife, Clinical Research Network South London), Maternity Voices Partnership Greenwich, Maternity Voices Partnership Lewisham, Ms Jenny Dorrington and the team at HOLOGIC, UK, West of England Academic Health Science Network.
References
Footnotes
Twitter @naomihcarlisle
Contributors NC, HAW and AHS all wrote the funding application and secured funding for this project. NC and HAW coordinated the development of the toolkit. NC produced the first and second draft of the toolkit, which was approved by HAW and AHS. NC wrote the first draft of this manuscript which was approved by HAW and AHS.
Funding The development of the QUiPP App was funded by the Guy’s and St Thomas’ Charity (Registered Charity No. 1160316) and Tommy’s (1060508). The development of the QUiPP App Toolkit was funded by a Health Innovation Network South London ‘Innovation Award 2019/2020’ awarded to: Ms Naomi Carlisle, Dr Ellie Watson and Professor Andrew Shennan.
Competing interests AHS is Principal Investigator on Hologic funded science grants, which are paid directly to institute. NC received financial assistance from Hologic covering expenses only, paid directly to institute, to provide educational talks on preterm birth.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.