Article Text
Abstract
Background In 2017, a provincial health-system released a Rehabilitation Model of Care (RMoC) to promote patient-centred care, provincial standardisation and data-driven innovation. Eighteen early-adopter community-rehabilitation teams implemented the RMoC using a 1.5-year-long Innovation Learning Collaborative (in-person learning sessions; balanced scorecards). More research is required on developing, implementing and evaluating models of care. We aimed to explore experiences of early-adopter providers and provincial consultants involved in the community-rehabilitation RMoC implementation in Alberta, Canada.
Methods Using focused ethnography, we used focus groups (or interviews for feasibility/confidentiality) and aggregate, site-level data analysis of RMoC standardised metrics. Purposive sampling ensured representation across geography, service types and patient populations. Team-specific focus groups were onsite and led by a researcher-moderator and cofacilitator. A semistructured question guide promoted discussions on interesting/challenging occurrences; perceptions of RMoC impact and perceptions of successful implementation. Focus groups and interviews were audio-recorded and transcribed alongside field notes. Data collection and analysis were concurrent to saturation. Transcripts coding involves collapsing similar ideas into themes, with intertheme relationships identified. Rigour tactics included negative case analysis, thick description and audit trail.
Results We completed 11 focus groups and seven interviews (03/2018 to 01/2019) (n=45). Participants were 89.6% women, mostly Canadian trained and represented diverse rehabilitation professions. The implementation experience involved navigating emotions, operating among dynamics and integrating the RMoC details. Confident, satisfied early-adopter teams demonstrated traits including strong coping strategies; management support and being opportunistic and candid about failure. Teams faced common challenges (eg, emotions of change; delayed data access and lack of efficient, memorable communication across team and site). Implementation success targeted patient, team and system levels.
Conclusions We recommend training priorities for future teams including evaluation training for novice teams; timelines for stepwise implementation; on-site, in-person time with a facilitator and full-team present and prolonged facilitated introductions between similar teams for long-term mentorship.
- implementation science
- rehabilitation
- health policy
- health professions education
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Background
Rehabilitation aims for ‘enhancing function for meaningful living’.1 In April 2017, a redesigned Rehabilitation Model of Care (RMoC) was introduced in a provincial health system to cultivate a patient-focused, innovative, equitable and data-driven service model.1–6 This coincides with the refrain ‘no decision about me without me’7 8 that undergirds many global, national and provincial movements to better engage patients in their health, healthcare and research.8–12
Models of care and their implementation
Understanding the implementation of policy innovations, like the RMoC, is critical to understanding change within complex health systems. Such understanding will build new knowledge for health systems, particularly on why models of care work in a particular jurisdiction and factors that influence model outcomes between jurisdictions.13 14 There is a call for more research on developing, implementing and evaluating models of care.15
A model of care is an ‘… evidence-informed policy or framework that outlines the optimal manner in which condition-specific care should be made available and delivered to consumers at a system level’.15 It is not a clinical guideline but a vehicle for moving best evidence into practice using appropriate teams, timing and resources.15 16 In Australia, Canada, the USA and the UK, models of care are increasingly developed for diverse settings including chronic conditions,17 18 e-health strategies,19 20 mental health20 21 and musculoskeletal issues.15 22 23
Research on perceived barriers and facilitators to implementing new models of care found that sustainable change is challenging.15 22–25 These facilitators included organisational cultures and stability; champions for change; individual acceptance; supportive leadership; distribution of decision-making roles and systematic follow-up and measurement.17 21 24 25 Recognised barriers included communication barriers; intraorganisational competition (eg, lack of formalised collaboration); organisational structures; lack of buy-in; limited financial resources; overwhelmed or unsupported staff.17 18 20 21 24 This research spans clinical settings including musculoskeletal conditions but does not address factors unique to community rehabilitation (eg, predominantly allied-health providers, outpatient settings).15 17–25
Implementation science theory supports understandings around why models of care work in a particular jurisdiction and clarifying factors to consider when spreading interventions across jurisdictions.13 14 The Consolidated Framework for Implementation Research (CFIR) represents a framework and ‘overarching typology’ to understand implementation developed from combining common constructs from published theories.14 26 The CFIR describes 5 domains, with a total of 37 constructs across the domains, that influence implementation effectiveness in multiple, complex ways individually, interactionally and collectively.26 The five domains relate to characteristics of the intervention (eg, ‘core components’ vs ‘adaptable periphery’ of the intervention; the intervention’s complexity, costs, course and evidential base); the outer setting (eg, network with external organisations, peer pressure, patient needs and resources and external policies or incentives); the inner setting (eg, internal structural characteristics, communication, climate and culture); characteristics of individuals (eg, self-efficacy, knowledge/attitudes/beliefs, personal attributes; individual state of change) and the implementation process (eg, the activities of planning, engaging, executing and reflecting and evaluating).26 This framework supports strategic assessments of potential barriers and facilitators to the implementation of a novel innovation, including a model of care.13
Organisational context
The RMoC has five competency domains: access and wayfinding; service options, client and community outcomes, transitions and professional practice (online supplemental appendix 1).4 The RMoC mandates standardised tools to capture collaborative goal-setting and patient-reported outcomes, including quality of life (EQ-5D-5L) and care experience (WatLX).27 28 This will inform implementation, policy development, quality improvement, accountability, comparisons and research.
Supplemental material
RMoC adoption began in May 2017: 18 community rehabilitation teams volunteered as early adopters. Early adopters then implemented the RMoC while being stewarded through the 1.5-year Innovation Learning Collaborative change-management process. These early-adopter teams represented multidisciplinary teams providing outpatient rehabilitation care, which was either general or specialised (eg, a balance programme, postoperative hip and knee rehabilitation and neurological rehabilitation). The represented disciplines included physiotherapy, occupational therapy, rehabilitation nursing and speech and language pathology. Based on the Institute for Healthcare Improvement’s Collaborative Model, the Innovation Learning Collaboratives gave structure and process to engage teams in change management.29–34 The Innovation-Learning-Collaborative process involved several strategies including independent study, team-based learning, face-to-face learning sessions and team-driven balanced scorecards for progress measurement.29
Separate from the RMoC, the provincial health system broadly introduced HealthChange Methodology.35 HealthChange focuses on educating providers to help patients make behaviour changes for health promotion.36 HealthChange training discusses person-centred approaches to patient engagement that may influence shared decision-making or collaborative goal-setting.36 The 18 early-adopter teams implementing the RMoC had priority to participate in the training.
Gaps in understanding
The literature around the development, implementation and evaluation of models of care is quite nascent, particularly in community rehabilitation. We aimed to contribute clarity on the implementation of novel intervention, particularly a model of care. We specifically aimed to clarify provider and professional experiences of implementation with the early adoption of the RMoC in Alberta, with a theory-informed analysis guided by the CFIR. This study is part of a broader research programme; other manuscripts described patient and provider perspectives on shared decision-making before RMoC implementation.37
Methods
We used focused ethnography in this research programme.38 Ethnography involves making cultural inferences from peoples’ communications, actions and artefacts.39 The culture of interest included patients and professionals composing diverse community rehabilitation sites across Alberta. Focused ethnography uniquely focuses on specific problems and contexts; on discrete social phenomena; on a single researcher’s conceptual orientation; on small samples; on limited to no participant observation and on academic and healthcare settings.40 41
Study sites or teams included those involved in enacting, directing or supporting the early adoption of the new RMoC between April 2017 and June 2018. Purposive sampling ensured diversity, particularly in patient populations and geographically, while accommodating feasibility considerations. Specific strategies included ensuring representation from urban and rural regions; including diverse array of early-adopter programme foci (ie, group-based and 1:1 care strategies; content specific vs general outpatient rehabilitation) and ensuring representation across the five zones of the health system organisation. As there were 18 early-adopter teams involved in RMoC adoption, all were invited and teams were followed up with to ensure maximum variation sampling.
Inclusion criteria for participants, whether providers, leadership or consultants, were either recognised membership on an early-adopter community rehabilitation team or a professional role facilitating RMoC implementation. Rehabilitation providers must have held a professional license, as appropriate, during implementation. No exclusion criteria were set.
Site leadership informed provider recruitment strategies. Tactics included email introductions followed by study presentations (by webinar, in-person or one-on-one) overviewing aims, methods and implications. After discussions with the previously unknown researcher, informed consent was procured.
Data collection
We used focus group methodology.42 43 Each focus group was limited to members of that team. Participants were offered the alternative of individual interview participation, if preferred for confidentiality or scheduling. We examined aggregate standardised-metric data (ie, EQ-5D-5L and WatLX) collected by early-adopter teams during the Innovation-Learning-Collaborative period (July 2017 to November 2018) and located on the provincial health-system’s cloud-based data-visualisation programme.
Site managers worked with staff to organise focus group timing and location on-site in private rooms. The provincial-consultant focus group used video discussions due to geography. Prior to the focus group, all participants received the written consent form, focus group guidelines and outline document, study backgrounder and a confidentiality agreement. The experienced, PhD-trained lead researcher (KPM) moderated all focus groups; a second research personnel acted as cofacilitator to log non-verbal behaviours and group dynamics in field notes.43 Due to geography and cost, the cofacilitator varied and included either a hired clerical staff, therapy assistant, patient-researcher or research trainee.
Focus groups were guided by a semistructured question guide. Discussion centred on participants’ experience of RMoC implementation; interesting or challenging experiences during implementation; perceptions of the RMoC in practice and criteria for defining successful RMoC adoption. The question guide was informed in form and content by ethnography and implementation science literature (particularly the CFIR described above), respectively.
Prior to data collection, previous Phase 1 provider-participants gave feedback on the question guide. The moderator convened the focus group, beginning with an ice-breaker and introduction. The moderator used verbal and non-verbal approaches (eg, calling on quieter participants, using head nodding and eye contact) to encourage participation.43 All focus groups and interviews were audio-recorded and confidentially transcribed.
Both the process of collection and the outcomes in the three standardised-metrics provided important insight into the issues related to success and sustainability of RMoC adoption. Primary data collection was novel for teams. We gained secondary access to the aggregate data on the provincial health-system’s data-visualisation platform specifically on the three standardised metrics (EQ-5D-5L, WatLX and Collaborative Goal Setting) for the period of the Early-Adopters’ Innovation-Learning-Collaborative (April 2017 and November 2018).
Data analysis
Data collection and analysis of field notes, transcripts and any participant notes were concurrent until saturation.38 39 Analysis began by uploading cleaned transcripts into NVivo, with coding of transcripts for words and phrases related to implementation, including experiences, successes and challenges. The research-trainee cofacilitator examined three coded transcripts for appropriateness and no missing codes. Similar ideas were grouped together to form themes, with tentative relationships among identified themes. This qualitatively derived description of the implementation experience of early-adopter teams and leadership was contextualised by descriptive analyses of site-level data including standardised RMoC metrics. An audit trail of decisions was kept to ensure rigour.44 The CFIR framework did provide an initial framework for qualitative data analysis but was deviated from so that the qualitative data content itself drove analysis.
Data analysis also considered the unique attributes of focus group research, particularly participant interaction.45–47 Coding was informed by the nuances of focus group interactions, including an examination of the sequence of responses to determine the process of evolving consensus and debate; an appreciation of individual contributions along the group discussion and an exploration of the impact made by types of questions (eg, general vs specific; particular topics).47
We used the provincial health-system’s data visualisation platform to analyse early-adopters’ aggregate data and SPSS 25 for the provider sociodemographic data collected in focus groups. For each site, we analysed the quantitative data to consider the collection process and outcomes related to the standardised metrics: EQ-5D-5L, WatLX, and collaborative-goal-setting and site characteristics (eg, disciplines, number of patients per month). We statistically described these data using means, SD and ranges for continuous variables (eg, number of patients) and proportions for categorical data (eg, types of disciplines). We used a monthly time series plot for the number of metrics captured for each standardised metric over the April 2017 to November 2018 period (three variables on one plot, each with a different line). The consolidated criteria for reporting qualitative research (COREQ) were used (online supplemental appendix 1).
Patient
The research question and methods were informed by discussions of the research team, key knowledge users and the two patient advisors who were trained in patient-directed research methodology. The patient advisors took part in all team meetings and also had separate meetings with the lead researcher to discuss study design, implementation, analysis and dissemination. Patient perspectives on the impact of the RMoC were examined in complementary research studies to the study described herein.
Results
Participant information
Ten of the 18 early-adopter teams participated in this study as well as a provincial team of community rehabilitation senior practice consultants. Forty-seven professionals participated in focus groups or interviews (30–120-min duration). The researchers conducted 11 team-specific focus groups (n=2–7 participants per group) and seven one-on-one interviews. Save the provincial consultant-team, all focus groups were in-person. Interviews were by phone (2) or in-person (5). Saturation was achieved.
Five teams represented metropolitan-urban settings, one team represented regional-urban settings and four represented rural settings. While the Innovation-Learning-Collaborative process began in May 2017, teams varied on when they initiated the novel, RMoC-required data collection processes. From the available data from eight teams, three teams began collecting the standardised metrics data in July 2017, two in August 2017, one in September 2017, one in October 2017 and one in November 2017. RMoC data collection initiation was not associated with geographic setting or survey delivery format (ie, paper vs iPad vs both). We found that mean completion rates were 87.25% at sites using paper-copies only and 95% at sites using both iPad and paper copies.
The mean (SD) age of the professional-participants was 41.4 (11.6) years, with the range being 45 years (youngest age 26 years and oldest 71 years). Most professionals were women (89.6%), Caucasian (78.7%), trained in Canada (85.1%) and worked in a hospital-based outpatient setting (61.7%). They had a mean (SD) of 16.2 (10.3) years of professional experience. Participants represented all three geographical areas: metropolitan-urban sites (53.3%), regional-urban sites (17.0%) and rural sites (29.7%). Diverse disciplines were represented including occupational therapy (25.5%), physiotherapy (23.4%), speech language pathology (10.6%), therapy assistants (21.3%), rehabilitation nursing (4.3%), respiratory therapy (4.3%) and social work (4.3%). The populations served by provider-participants included patients with older age (27.7%), neurological issues (25.5%), chronic health issues (17.0%), homecare needs (4.3%) and musculoskeletal issues (4.3%). About 66% of providers describe the primary service option under the early-adopter team as a group-based programme.
We examined the WatLX completion rates and actual measures at the available eight sites. Across July 2017 to November 2018, on average 85% of patients responded entirely agree or mostly agree on the 10 individual WatLX items. Individual items that had a higher response rate for not-applicable related to inclusion of chosen family and friends (32%), control of physical pain (15%) and no delay on information availability (13%).
We examined the EQ-5D-5L completion rates and actual measures at nine available sites between July 2017 and November 2018. Sites collected 1376 intake EQ-5D-5L surveys and 753 end-of-episode-of-care EQ-5D-5L surveys. In July 2017, the ratio of intake EQ-5D-5L surveys completed to end-of-care EQ-5D-5L surveys was 86.61%–13.39%. In November 2018, this ratio was 64.36% intake and 36.54% end-of-care surveys. Across the nine sites, the mean change in EQ-5D-5L Index Score was 0.11 in July 2017 and 0.09 in November 2018. The largest monthly mean change in EQ-5D-5L Index Score was 0.14 and the lowest monthly mean change was 0.04: these are all above the minimally important difference of 0.037.48 The mean number of patients who indicated that they had no problem across the five EQ-5D-5L dimensions was 31.80% at intake and 41.27% at the end of care.
Professionals’ experience of RMoC implementation
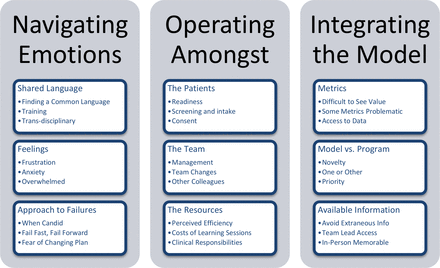
The experience RMoC implementation called on teams to navigate emotions, operate among myriad dynamics and integrate novel RMoC processes (figure 1). Professionals described implementation success as multifaceted, relating to patient metrics, team processes and system efficiency (figure 2).
Early-adopter professional experiences during RMoC implementation in community rehabilitation. RMoC, Rehabilitation Model of Care.
Early-adopter providers’ perceptions of success as multifaceted. RMoC, Rehabilitation Model of Care.
Navigate emotions
The emotional fallout of RMoC implementation was prominent. Emotions and connections were intertwined for providers. Many providers spoke of developing a common, shared language during implementation, which was facilitated through training (eg, HealthChange) and transdisciplinary approaches. All teams experienced feelings of frustration, anxiety and being overwhelmed, especially at the start. Failure, or when things did not go as planned, often caused stress, challenges and other negative emotions at the individual level and team level. Teams varied in their approach to failure. Some teams were open and candid about failure and took an opportunistic ‘fail fast, fail forward’ approach. This mitigated stress and pre-empted failure-related delays. Other teams were hesitant to change plans with unexpected events. Table 1 contains exemplar quotes.
‘[HealthChange] gave us the language I think right. We were able to have easier conversations around our clients because HealthChange gave us the lingo, the terminology so that we were all on the same page.’ (Focus Group 4 Participant)
‘So that first session was very frustrating admittedly. It was very confusing. It was hard to understand what the ultimate goal was and what we were expected to achieve at the session. So when we got to the end of the session and … they’re looking to me essentially to make some decisions as to what we’re doing, and I’m like ‘I still really don’t understand the question.’’ (Focus Group 8 Participant]
Professional quotes on navigating emotions in the RMoC implementation experience
Operating among dynamics
Early-adopter teams faced challenges and made adaptions for microlevel, mesolevel and macrolevel dynamics. At the microlevel, patient–provider interactions were routed towards emphasis on patient readiness and understanding for fully informed participation. This was especially prominent during screening and intake. At the mesolevel, interactions among team members influenced implementation. Management support determined the relative ease of implementation. Change in team membership was a common challenge. At the macrolevel, the use and availability of organisational resources was critical to professionals. Perceived inefficiencies in resource use, particularly the four in-person Learning Sessions (as part of the Innovation-Learning-Collaborative), concerned professionals, as did any unavailability or inconsistency in resources including time, physical and human resources. Table 2 provides transcript quotes.
‘Yeah, lots of mat leaves and coverages and people leaving early and so we were in scramble mode for the last year just trying to get through our day in peace.’ (Focus Group 4 Participant)
‘P1: We’ve always struggled with that and people have great ideas about how to help us with our problems. But that’s because they have all these resources and funding …
P2: I think like the community support right. A lot of those programs are run off site at gyms and there’s collaborative partnerships, which we don’t have.’ (Focus Group 1 Participants)
Professional quotes on operating among dynamics in the RMoC implementation experience
Integrating the model
Three aspects related to the integration of RMoC polices and processes. First, some teams questioned introducing data collection with standardised and non-standardised metrics. It was difficult to see the data’s value. Some metrics were problematic due to ceiling effects or inapplicability to unique populations. Prolonged delays in access also limited data utility.
Second, RMoC implementation was accompanied by either novel service programming or an opportunity to highlight provincially unique, long-standing site services. Some teams inappropriately conflated the RMoC and service programming. These struggles and misunderstandings of the RMoC led to lower prioritisation to implementation and integration activities.
Third, RMoC integration was tied to information availability. Some team members avoided extraneous information in their daily tasks, which sometimes included RMoC-related information. Team leads had the greatest connection to the RMoC, in contrast to the team. Training and learning opportunities were most memorable and impactful when in-person, practice-relevant and resource-efficient. In-person interactions that were perceived as inefficient or irrelevant were both unmemorable for content and associated with negative attributes. Table 3 contains quotes supporting these features.
‘The model of care was not first and foremost in my mind, ever. I’m sorry to say… But basically when you’re on a floor working with clients, you’re trying to get your day to day done. Clients need to be seen. They need to be heard. Sometimes I think I have a second mom role.’ (Focus Group 2 Participant)
‘It seems like we retained the in-person stuff a lot better. … The practical side of it… It was much more useful, much more effective so.’ (Focus Group 3 Participant)
Professional quotes on integrating the model during RMoC implementation experience
RMoC implementation towards multifaceted success
Professional participants described successful implementation as multifaceted, not monolithic. Success fell across microlevels, mesolevels and macrolevels. Success was measured in improvements in patient experience and outcomes, as measured by the standardised RMoC metrics. Team processes tracked success, particularly whether the team was cohesive, collaborative and resilient through the inevitable challenges of implementation. RMoC success was tied to whether implementation introduced efficiencies for the health system.
‘I don’t think metrics is the only way to measure success. … I think there also needs to be discussion about how we felt about the successes we had. To take on all of those changes and come out like a year later.’ (Focus Group 4 Participant)
‘I think success is not just getting to a spot and being happy with it. It’s constantly re-looking at things and making those changes to make it even better and better all the time.’ (Interview 2 Participant)
Discussion
Practical implications
Understanding the professional-participants’ experience as early adopters of the RMoC clarifies commonalities. First, the features and framework of the implementation experience house a list of common challenges (online supplemental appendix 2). Clarity on those challenges can help prepare future teams, and the leadership supporting them, on what to expect during RMoC implementation. These challenges included:
Supplemental material
difficulty in handling and navigating the initial emotions of large-scale change, which stressed team members and affected motivation,
lack of timely data access,
misunderstanding the relevance of RMoC metrics,
lack of efficient, memorable updating and training strategies for teams,
lack of clarity on the RMoC aim, vision and key components,
lack of sufficient time and coverage for clinical responsibilities to complete implementation tasks,
perceptions of uniqueness impeding collaborative and mentorship opportunities.
Some early-adopter teams had a fraught implementation experience. Other teams described an experience marked with confidence and satisfaction. These confident teams had four features in common. They had coping strategies that pre-empted delays on management or team-member changes (eg, strong communication, human resources to supplant gaps as new members came up to speed). They used the RMoC and HealthChange strategies to build a common language that facilitated communication among the team, with external colleagues, and with patients. They consistently addressed failure with candour and opportunism. They felt tangible management support for implementation tasks and less top-down decision-making.
We developed recommendations for RMoC spread (online supplemental appendix 3). These recommendations highlight educational priorities (eg, evaluation training); inevitable experiences to prepare for (eg, being overwhelmed and anxiety); examples and strategies (eg, a timeline to adopt the RMoC in sequential parts) and the optimal format for education (eg, efficient, in-person sessions with the full team present).
Supplemental material
Contextualising findings
These findings corroborate many empirically recognised facilitators and barriers to the implementation success of models of care. Facilitators-wise, we saw that individual acceptance, supportive leadership, distribution of decision-making roles and the power of systematic measurement and sustainability were critical.17 21 24 25 Barriers-wise, limited resources, lack of buy-in, communication and overwhelmed or unsupported staff were present in Alberta as in other jurisdictions that struggled with model-of-care implementation.17 18 20 21 24 We move beyond this extent literature in several ways.
First, previous research emphasised organisational structures that work to impede or facilitate model-of-care implementation.17 18 20 21 24 25 Our findings focus less on organisational or policy factors and rather emphasise the importance of the interpersonal factors, particularly emotional and communicative factors. In the language of the CFIR,26 the intervention and individuals involved with the intervention were more determinative of implementation, while the inner and outer settings as well as the process of implementation were less prominent.
Individual connection to, and clarity about, the RMoC was important. The navigation of emotions was a conspicuous process in the implementation experience. Teams that struggled in implementation generally got ‘stuck’ and could not steer the emotional fallout of large-scale change, novel transdisciplinary approaches and dynamic team membership. Lack of connection meant lack of motivation and successful RMoC implementation was distant. Teams could neither get behind increased data collection nor data-driven innovation.
The RMoC—the intervention itself—had adaptable and requisite components.26 The adaptable components sometimes exaggerated confusion around RMoC vision and aims. Early adopters could select the patient population and type of service programming on which to apply the RMoC components. This selection was informed by local needs, interests and available resources. Where teams introduced novel service programming and were somewhat unsure, the new programme (eg, a new group programme for balance) and the RMoC became conflated. In implementing models of care, the challenges consequent to the adaptable and requisite interventional components must be made explicit so that they can be addressed.
Second, this study addresses the call to understand why models of care work in a particular jurisdiction and the factors that influence outcomes when transferring between jurisdictions.13 14 Through the common challenges and the characteristics of confident teams, we see jurisdictional lynchpins. The size, dynamism, resources and attitudes of all rehabilitation staff at a particular site informed the subset, Early-adopter team success in realising the RMoC facets and aims. Rural teams struggled more often with fewer resources, smaller teams and less sustained reprieve from clinical duties to spend the time required to understand the RMoC and implement its component parts.
Third, this study confirms the importance of sustainability considerations in model-of-care implementation. The training associated with the RMoC (along with HealthChange) likely offers gains in shared decision-making and functional goal-setting quality and frequency. These gains can be lost. Challenges to sustainability include competing responsibilities, lack of clarity or connection to the RMoC vision, less traditional clinical settings and team dynamics. Teams adopting the new models of care must consider team and patient strategies for sustainability. For example, after training courses are complete, teams must plan the logistics of developing a community of practice that carries the conversation forward around the learnings.
Limitations
We recognise our study limitations. Pre-existing team dynamics carried into the focus group and may have affected candour and communication styles. No managers participated in focus groups, but team leads were present. Not all focus groups occurred at the same time of day or same time since implementation. The latter may lead to different levels of recall or acceptance. The former seemed influential as afternoon focus-group participants were quieter and less-forthcoming. Some interviews lacked the elaboration of focus groups. Ethically and feasibly, interview opportunities were necessary. Given there was little dissent and infrequent disagreement among focus group participants, it suggested general consensus.
Conclusion
This study has organisational relevance to health systems aiming to use models of care as frameworks that can advance patient-centred care in allied-health-dominant domains. We clarify the professional experience of early adopters of the RMoC, which provides a foundational information resource to expose seminal differences between jurisdictional success or failure in implementation. Possible future research directions include (a) rigorous development, testing and implementation of the training strategies identified and (b) evaluating the RMoC itself using research designs that acknowledge and measure fidelity.
Acknowledgments
We acknowledge our participants from the early-adopter teams as well as consultants and leadership at Alberta Health Services. We acknowledge the site managers and non-participating staff members at each site who were facilitated participant time away from clinical responsibilities to take part in this study. We thank our Patient and Community Engagement Researchers (PaCERs), Jean Miller and Sylvia Teare, for their advice, insight and support in the broader research programme on shared decision-making in community rehabilitation. We particularly thank the provincial and regional leadership at Alberta Health Services, including Lisa Warner and Elaine Finseth, who were (and continue to be) champions of this work.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @Kiran.Manhas2
Contributors KPM helped contribute to the study’s conceptualisation and design, implemented the study methodology, managed resources and developed all manuscript drafts. KO helped contribute to the study’s conceptualisation and provided ongoing supervision in close collaboration with the other senior authors (SV, TW) regarding its methods, conduct, analysis and manuscript development. KC helped contribute to the study’s operationalisation; provided ongoing support on conduct, analysis and manuscript development and reviewed and edited this manuscript. SV helped contribute to the study’s conceptualisation and provided ongoing supervision in close collaboration with the other senior authors (KO, TW) regarding its methods, conduct, analysis and manuscript development. TW helped contribute to the study’s conceptualisation and provided ongoing supervision in close collaboration with the other senior authors (KO, SV) regarding its methods, conduct, analysis and manuscript development.
Funding This work was supported by the Canadian Institutes for Health Research Health System Impact Fellowship (Code 201705HI7-388576-170744, 2017). Supplemental funding was provided by the Strategic Clinical Networks and Research Challenge portfolios at Alberta Health Services.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study was approved by the Conjoint Health Research Ethics Board, University of Calgary (REB18-0967). All participants provided written informed consent prior to participation.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement No data are available. Because of the inability to deidentify qualitative data, the data from this study are not publicly available for open or limited sharing.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.