Article Text
Abstract
Paediatric haematology, oncology and bone marrow transplant (BMT) patients frequently require transfusion of blood products. Our institution required a new transfusion consent be obtained every admission. The objectives of this project were to: revise inpatient blood products consent form to be valid for 1 year, decrease provider time spent consenting from 15 to <5 min per admission, and improve provider frustration with the consent process. Over 6 months, we determined the average number of hospitalisations requiring transfusions in a random sampling of haematology/oncology/BMT inpatients. We surveyed nurses and providers regarding frustration levels and contact required regarding consents. Four and 12 months after implementation of the annual consent, providers and nurses were resurveyed, and new inpatient cohorts were assessed. Comparison of preintervention and postintervention time data allowed calculation of provider time reduction, a surrogate measure of improved work efficiency. Prior to the annual consent, >33 hours were spent over 6 months obtaining consent on 40 patients, with >19 hours spent obtaining consent when no transfusions were administered during admission. Twelve months after annual consent implementation, 97.5% (39/40) of analysed patients had a completed annual blood products transfusion consent and provider work efficiency had improved by 94.6% (>30 hours). Although several surveyed variables improved following annual consent implementation, provider frustration with consent process remained 6 out of a max score of 10, the same level as prior to the intervention. Development of an annual inpatient blood products consent form decreased provider time from 15 to <1 min per admission, decreased consenting numbers and increased work efficiency by >90%.
- blood component transfusion
- bone marrow transplantation
- efficiency
- hematology
- neoplasms
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Problem
In the paediatric haematology, oncology and bone marrow transplant (BMT) outpatient clinics at Texas Children’s Hospital/Baylor College of Medicine in Houston, Texas, USA, patients and families have the option to sign consent for blood products administration that is valid for an entire year. In contrast, however, a new consent for blood products transfusion was required on each inpatient admission for these patients. From the patients’ and guardians’ perspectives, frustrations due to repeatedly signing the same consent on concurrent admissions contributed to consent fatigue. The process consumed a significant amount of provider time. Furthermore, blood transfusions were often delayed while nurses waited for providers to obtain parental consent for blood products administration. This lead to decreased nursing work efficiency, decreased nursing satisfaction and worsened nurse–provider collaboration and communication.
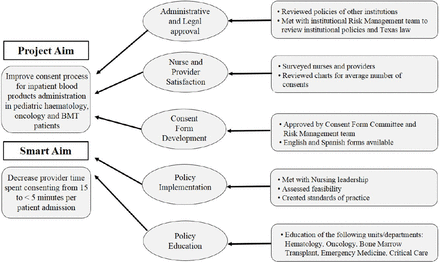
Project goals were to decrease the burden of consents in the chronically transfused paediatric haematology, oncology and BMT population; improve nursing and provider satisfaction; and increase inpatient work efficiency by reducing the time spent obtaining consent for blood products administration. To reach this goal, our team of fellow and attending physicians instituted a new hospital-wide annual blood products consent form for hospitalised paediatric haematology, oncology and BMT patients. The overall aim of this quality improvement project was to change the current blood products consent process for haematology, oncology and BMT patients admitted to our hospital to allow the option for inpatient blood products consent to be valid for 1 year. Our project’s SMART aim was to decrease provider time spent consenting from 15 to <5 min per patient admission (figure 1). An additional project goal was to improve provider frustration with the blood products consent process over a 1-year period.
Background
In the United States, over 5 million people receive blood products transfusions each year.1 Within the field of paediatrics, haematology, oncology and BMT patients frequently require inpatient transfusions of blood products.2 Informed consent for blood products transfusion is recommended by the American Association of Blood Banks, US Joint Commission and UK’s Advisory Committee on the Safety of Blood, Tissues and Organs.1 3–7 At our institution, over 500 new oncology patients and 650 new haematology patients are seen each year. As an academic free-standing children’s hospital, most of our patients are presented with the option to participate in clinical research trials, from phase III Children’s Oncology Group treatment protocols to institutional basic science studies requiring patient blood samples. However, there was increasing concern that the sheer volume of treatment, procedure and research consents could lead to ‘consent fatigue’. As an example, families of patients with newly diagnosed pre-B-cell acute lymphoblastic leukaemia, the most common paediatric oncological diagnosis,2 are presented with a minimum of 15 consent forms in the 5 weeks following initial diagnosis. Our quality improvement team began evaluating the process of consent for opportunities to decrease the consent burden on patients and families without adversely affecting either patient care or research efforts.
Measurement
Phone interviews were conducted with 12 large academic children’s hospitals around the USA to determine their standard of care regarding blood products consents for paediatric haematology, oncology and BMT patients. Eighty-three per cent (10/12) of the hospitals contacted required that inpatient blood products consent be obtained on this chronically transfused patient population either annually or only once at the time of disease diagnosis, and subsequently remaining valid for the duration of the patient’s treatment at that hospital until the age of 18 years. To better determine the local feasibility of this process, another comprehensive cancer centre in Houston was polled to assess their standard of practice. This institution required blood products consent be signed only once for the duration of a patient’s treatment either in the inpatient or outpatient settings.
In order to determine how much time was spent by providers in obtaining inpatient blood products consents, our team assessed the average time required to obtain a blood products consent at our institution. This measurement took into account the time needed for the nurse to note the lack of consent and contact a provider, need for a translator, time required to find or contact the patient’s guardian, time required to find and fill out the consent form, and the consenting process itself. We subsequently determined the average number of inpatient hospital admissions requiring blood products transfusion in a random sampling of 40 haematology, oncology and BMT inpatients over a 6-month period. We retrospectively tracked a random sampling of 40 patients over a 6-month period in order to gain a real-time assessment of transfusion practices and needs. With 3000 admissions/year, random sampling was appropriate to represent an adequate cross-section of our inpatient population, especially given the wide variation in diagnoses seen within this diverse patient population as well as the progression of patients through treatment (with variable transfusion requirements on each inpatient admission). With these data and the measured time needed to obtain a blood products consent, we were able to calculate the time spent by providers in obtaining inpatient blood products consent. Additionally, as the section policy is that blood products consent should be obtained for all haematology, oncology and BMT patients on each admission, we calculated the time spent obtaining blood products consent in this patient population for admissions in which blood products were not required.
We next queried inpatient haematology, oncology and BMT nurses with a seven-question survey to determine the effort they expended in having to repeatedly request that blood products consents be obtained by providers, delays in blood product administration due to lack of blood products consent and how often a blood products consent had already been completed at the time blood products were ordered. Physicians at the resident, fellow and attending levels, as well as advanced practice providers (APPs), were also polled with a six-question survey to assess current blood products consent practices, frustration level and time expenditure.
Design
This ‘Model for Improvement’-type quality improvement project was performed at Texas Children’s Hospital (TCH), Baylor College of Medicine (BCM), the largest children’s hospital in the USA.8 This project was classified as ‘Model for Improvement’ as the end goal is to expand the annual blood consent to cover all chronically transfused patients seen in both inpatient and outpatient encounters at all three TCH campuses. However, we began with the following small-scale testing in inpatient paediatric haematology, oncology and BMT patients. This project meets three of the six Institute of Medicine Quality Dimensions: timeliness, efficiency and patient-centred. The key drivers included hospital administrative and legal approval, nurse and provider satisfaction, consent form development, and policy implementation and education (figure 1). We consulted with the TCH Transfusion Committee as well as paediatric nursing leadership, risk management and legal teams in order to obtain institution-wide approval for this quality improvement project. A new consent form entitled ‘Annual Blood Consent: Transfusion of Blood or Blood Products Disclosure and Consent/Refusal (Inpatient), Haematology/Oncology/Bone Marrow Transplant Use Only’ was then created in both English and Spanish and approved by the TCH Forms Review Committee. The consent form was printed on a gold paper to differentiate it from the standard inpatient blood products consent form valid only for the duration of one inpatient admission. New annual blood products consent forms were distributed hospital wide, including the critical care units and emergency department. The TCH Health Information Management staff were responsible at the time of a patient’s hospital discharge for scanning the paper consent into the electronic medical record so that the consent would be readily accessible by providers and nurses on the patient’s subsequent inpatient admissions. A new document type was created in the electronic medical record entitled ‘Annual Blood Consent’ to facilitate identification of the consent form on subsequent inpatient encounters.
Drivers diagram. SOP, standards of practice.
Strategy
The TCH blood transfusion nursing policy and procedure were updated with the aid of nursing leadership to reflect the change to an annual blood products consent option for paediatric haematology, oncology and BMT patients. Additionally, a new standard of practice document was created for providers to similarly reflect the new annual blood products consent form. Next, wide-scale educational efforts across the institution were undertaken to educate (1) critical care, emergency medicine, and haematology, oncology and BMT attending and fellow physicians and APPs; (2) paediatric interns and residents; (3) inpatient nurses and (4) unit secretaries on the new process changes (ie, new form, documentation requirements and filing location in electronic medical record). A variety of methods were used to disseminate information, including oral presentations in large and small groups, self-directed PowerPoint presentations, email correspondence and informational documents. Providers obtaining blood products consent were required to document the date of consent in the electronic medical record. To facilitate this documentation, an electronic SmartPhrase (with the date automatically populated) was created so as to ease completion by providers.
At the 4-month postimplementation time point, our aim was to determine if the new annual blood consent form was being implemented correctly and measure if the number of consents obtained per patient and provider time spent obtaining consents had decreased (audit cycle shown in figure 2). Consequently, after implementation of the new annual blood products consent form, providers and nurses were re-queried with surveys to assess for changes in time spent obtaining consent, administration delays in transfusion of blood products due to consent-related issues and frustration with the new consent process. Additionally, at 4 months postintervention, new random samplings of 40 haematology, oncology and BMT inpatients were performed (to decrease the risk of bias) to assess for changes in the number of blood products consents obtained as well as the time spent obtaining consents on these patients. A 4-month time frame was chosen for the first set of assessments rather than 6 months in order to provide an earlier assessment of potential barriers (if any) needing to be addressed. Of note, data obtained 4 months postintervention were normalised to a 6-month period for comparison purposes; using the 4-month postintervention data, we calculated the average number of consents obtained, number of hospital admissions, admissions requiring blood products and the estimated time spent obtaining blood products consent per month and then multiplied these values by 6 in order to normalise the data to a 6-month period. The data obtained at the 4-month postimplementation time point showed that there was some difficulty from a nursing standpoint in finding the annual blood products consent form in the medical record for some patients on subsequent admissions. The data also demonstrated decreases in both provider time spent obtaining blood consents and the total numbers of blood consents obtained. These results supported our overarching hypothesis that the institution would benefit from an annual blood consent.
At the 12-month postimplementation time point, our study aim was to determine if our initial hypothesis was correct by assessing for decreases in both provider time spent obtaining blood consents and the total numbers of blood consents obtained (audit cycle shown in figure 3). Survey and patient data were again gathered as described above at the 4-month postimplementation time point. Comparison of the postintervention time data with the preintervention data allowed calculation of time reduction by providers, as a surrogate measure of improved work efficiency.
Audit cycle #1: 4 months postimplementation.
Audit cycle #2: 12 months postimplementation.
Results
Admission volumes and numbers of blood products transfusions were retrospectively determined from a random sampling of 40 haematology, oncology and BMT inpatients over a 6-month period prior to implementation of the annual blood products consent form. Prior to implementation of the annual blood products consent, standard practice was to obtain a new transfusion consent form on each patient at the time of each hospital admission. In this patient sample, there were 139 admissions (and consequently blood products were consented for 139 times) over a 6-month period (median 24 admissions/month) (table 1). The average time spent by providers in obtaining consent for blood products transfusion was 15 min (median 340 min/month), therefore >33 hours were spent by providers over a 6-month period obtaining blood products consent. However, since only blood products were required in only 43% of these admissions (60/139), over 19 hours were spent by providers obtaining blood products consent when no transfusions were administered over the course of the inpatient admission. Of note, in the US healthcare system, time spend obtaining blood products consents is not considered billable time.
Data obtained retrospectively at each time point over a 6-month period from 40 haematology, oncology and BMT patients (data at 4-month time point extrapolated to six period for comparison purposes)
Four and 12 months after implementation of the annual blood consent, admissions and blood products transfusions data were retrospectively collected from a new random sampling of 40 haematology, oncology and BMT inpatients. The 4-month data were normalised to a 6-month period for ease of comparison. Numbers of admissions increased in the 4 months after implementation of the annual blood consent (median 42.5 admissions/month) and were similar to the preimplementation number during months 6 through 12 after consent implementation (median 20 admissions/month). The percentage of admissions in which blood transfusions were required were similar at all three time points (table 1). However, by 12 months after implementation of the annual blood products consent, 97.5% (39/40) of the analysed patients had a completed annual blood products transfusion consent in their electronic medical records. Consequently, the number of blood products consents and thus the average time spent obtaining consent per admission significantly decreased over time from median 340 min/month prior to consent implantation to only 30 min/month by months 6 through 12. By 12 months after implementation of the annual blood consent, provider work efficiency, determined by reductions in provider time spent obtaining blood products consent, had improved by 94.6%. Over time for three representative populations each comprised 40 inpatient haematology, oncology and BMT inpatients, the number of transfusions increased while the time spent obtaining consent decreased after implementation of the annual blood consent (figure 4).
Run charts demonstrating: (1) stable to increased numbers of hospital admissions, (2) increase in number of transfusions from prior to annual consent implementation and (3) decrease in time spent obtaining blood products consent over time for a representative panel of 40 paediatric haematology, oncology and BMT patients. Lines indicate median measurements over each time interval.
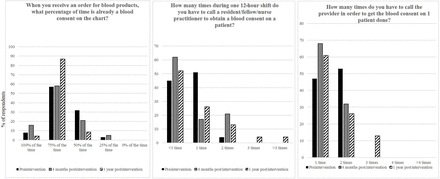
Surveys at all three time points were completed by >20 inpatient haematology, oncology and BMT nurses (figure 5) and providers (figure 6). Many variables improved following implementation of the annual blood products consent, including decreased phone calls by nurses to providers regarding the need for blood products consent. There was no change in provider frustration with obtaining a blood products consent, which remained at the same level as prior to the intervention, 6 out of 10.
Selected survey results of inpatient paediatric haematology/oncology nurses performed: (1) prior to implementation of the annual blood products consent, (2) at 4-month and (3) at 12-month assessments.
Selected survey results of paediatric residents, paediatric haematology/oncology fellows and inpatient paediatric haematology/oncology nurse practitioners performed: (1) prior to implementation of the annual blood products consent, (2) at 4-month and (3) at 12-month assessments.
Lessons and limitations
Early on after implementation of the annual blood products consent, nurses expressed occasional difficulty with finding the scanned consent form in the electronic medical record on a patient’s subsequent admissions, as the signed consent needs to be visualised by the nurse prior to initiating a blood products transfusion. This led to several patients requiring a provider to obtain a second annual blood products consent. To address this issue, TCH Health Information Management staff received re-education and reorientation to ensure that signed annual blood products consent forms were scanned into the electronic medical record and appropriately labelled on a patient’s hospital discharge. In order to sustain this intervention, paediatrics residents rotating on the haematology and oncology service are informed of the annual blood products consent form during the monthly orientation lecture at the start of their rotation. New haematology, oncology and BMT fellows and APPs are also made aware of the consent form each year during an orientation session. Offering of the annual blood consent form to haematology, oncology, and BMT patients and families and appropriate documentation of the consent is now considered mandatory for all providers caring for these patients. Nursing education regarding the annual blood consent form is not only performed hospital wide for newly hired staff but refresher education is provided throughout the year by blood bank staff and nursing educators.
The end goal of this ‘Model for Improvement’ project as the end goal is to expand the annual blood consent to cover all chronically transfused patients seen in both inpatient and outpatient encounters at all three TCH campuses. However, we first trialled this intervention with the above small-scale testing in only inpatient paediatric haematology, oncology and BMT patients.
This project focuses only on inpatient blood products transfusions in a specific paediatric patient population. However, the data are generalisable to both adult and paediatric patients in inpatient and outpatient hospital settings that receive chronic transfusions. Additionally, the project is relevant internationally at all sites with electronic medical records. This project is limited by the fact that data collection stopped 1 year following implementation of the annual blood products consent. The strengths of the project include the large number of patients seen at TCH, the excellent reduction in provider time spent obtaining consents following the intervention, and both the sustainability and planned spread of the project.9
Our data were retrospectively obtained in a representative population of 40 paediatric haematology, oncology and BMT inpatients, and we acknowledge that ideally this data collection would have been performed in a prospective manner. We additionally recognise that we did make several interventions in this project, and it is difficult to quantify which specifically are responsible for the noted improvements. Finally, as in all small studies, chance, bias and confounding could potentially have led to our results due simply to random fluctuations, especially in our nurse and provider survey data. However, the decreased time spent in obtaining consents noted after implementation of the annual blood consent is felt to be accurate as this decreased was noted despite stable numbers of admissions and increased numbers of transfusions over time (figure 4).
Conclusion
The aim of this quality improvement study was to create an annual inpatient blood products consent for haematology, oncology and BMT patients at our large, academic, tertiary care children’s hospital and thus decrease provider time spent obtaining blood products consent from 15 to <5 min per patient admission and improve provider frustration with the blood products consent process over a 1-year period. We were able to exceed our first goal, decreasing provider time per consent per admission from 15 min to <1 min. Unfortunately, we did not meet our second goal as provider frustration with the act of obtaining blood products consent remained unchanged. This is not unexpected, however, because our intervention did not change the process of obtaining blood products consent but only the frequency. Of note, the number of consents obtained decreased significantly with the implementation of the annual blood products consent, so the frustration felt by providers occurs less frequently.
Our intervention resulted in a 95% increase in provider work efficiency. This intervention can feasibly be implemented at other institutions that treat chronically transfused patients in order to decrease provider time. The implementation of the annual blood products consent also decreased the number of phone calls between providers and nurses related to obtaining consent. This surrogate marker may reflect improvement in haematology, oncology and BMT inpatient nursing–provider communication related to this issue.
Following initiation of the annual blood products consent form for haematology, oncology and BMT patients, other divisions within the institution that treat chronically transfused populations, such as cardiology, have expressed interest in expanding the patient populations covered by the consent. After obtaining approval to proceed from hospital to physician, nursing and administrative leadership at the main campus of TCH as well as the two satellite campuses, we subsequently met with physician leaders from various paediatric sections. Neonatology felt that their patient population would not greatly benefit from the use of an annual blood products consent. In contrast, critical care, emergency medicine and nephrology sections requested inclusion in the expanded programme. Additionally, our team met with hospital ambulatory and inpatient nursing leadership, the TCH Blood Bank and Transfusion committee, health information management, and the hospital’s risk management and legal teams. We are currently in the final stages of expanding the scope of the annual blood consent to cover all chronically transfused patients seen in both inpatient and outpatient encounters at every location within our integrated delivery system (ie, three different campuses).
In conclusion, development and implementation of an annual blood products administration consent form for inpatient paediatric haematology, oncology and BMT patient significantly increased provider work efficiency.
Acknowledgments
The authors appreciate the collaboration of the 12 children’s hospitals throughout the USA that was contacted in order to determine their standard of care regarding blood products consents for pediatric hematology, oncology and BMT patients. They additionally acknowledge the expert assistance obtained from the Center of Clinical Effectiveness at Baylor College of Medicine and Texas Children’s Hospital and specifically from Charles G Macias, MD.
Footnotes
Contributors HL: planned study design and implementation, provided nursing and provider education, participated in analysis of results and drafted the manuscript. SB: completed data analysis and reviewed the manuscript. CB, SS and SBW: conducted surveys and reviewed the manuscript. YL-K: assisted in study design and implantation and reviewed the manuscript. MDS: planned study design and implementation, provided nursing and provider education, and reviewed the manuscript.
Competing interests None declared.
Provenance and peer review Not commissioned; internally peer reviewed.