Article Text
Abstract
Background Cardiac rhythm devices (CRD) require complex management to identify potential device or patient issues. While easy to obtain, report processing is complex and time consuming. In our population, a majority of reports were performed outside of institutional protocols and no method for electrophysiology (EP) notification for unscheduled reports existed. These process breakdowns led to potential issues with safety and associated loss of work efficiency.
Objective Our aim was to decrease the percentage of reports without EP notification from 30% to 10% over a 9-month time period.
Methods We created a detailed process map of in-office and home device reporting. Failure mode and effects analysis (FMEA)/Pareto charts were used to determine the mechanistic underpinnings of notification failures and identify areas for process improvement. Multiple interventions were implemented using the Plan-Do-Study-Act (PDSA) technique. Process run charts and control charts were used to evaluate ongoing changes.
Results Our FMEA identified failures related to (1) lack of physician understanding of the device reporting system, (2) lack of an easy to use method of EP notification and (3) lack of patient understanding of report notification. Pareto charts identified the most frequent failures to be associated with specific cardiology subspecialties as well as reports sent from home. We performed multiple interventions including(1) creation of an easy to use method of EP notification used by patients and medical staff, (2) physician education and (3) patient education. Compared with baseline reporting, there was a decrease from 30% to <10% of device reports obtained without EP notification. This process improvement additionally resulted in a 34% reduction in time required for device processing.
Conclusions Development of a unified EP reporting system and quality improvement methodology resulted in improved CRD report notification and improved efficiency for staff. These process changes resulted in improvement across differing cardiac subspecialty providers and patients.
- control charts/run charts
- continuous quality improvement
- healthcare quality improvement
- pdsa
- quality improvement
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
- control charts/run charts
- continuous quality improvement
- healthcare quality improvement
- pdsa
- quality improvement
Problem
Cincinnati Children’s Hospital is a large, tertiary children’s hospital that follows approximately 450 paediatric and adult patients with cardiac rhythm devices (CRD). Our patient population is derived primarily from local and regional populations consisting of both private and government payors, with additional patients followed nationally. Many of these patients live outside the local geographic region, and current device technology allows for transmission of device reports from a patient’s home. Although the data are easy to obtain, the processing of CRD reports can be time consuming and involves timely identification of any patient or device-related problems.1 These problems can be subtle and without knowledge of associated patient symptoms and the reasons for device reporting, potential problems can be missed. The time needed to obtain this additional information is time consuming and inefficient. Institutional algorithms had been created with regard to timing of device reporting but the algorithm requires compliance from patients and managing physicians.2 A combination of recent increases in device implantation and utilisation of device reporting had led to significant increases in device processing time and strain on the system. In review of the 6-month time period prior to this project, approximately two thirds of patients were not reporting according to institutional algorithms, primarily with significant over reporting. Adding to the inefficiency of the system, a significant portion of device reports were sent from home or obtained in the office without notification to the electrophysiology (EP) service responsible for device management and reporting. Our SMART Aim was to decrease the percentage of CRD reports obtained without EP notification from 30% to 10% from June 2016 to April 2017.
Background
While prior studies have demonstrated improved quality of care and reduced healthcare use in specific populations such as adult heart failure patient populations, similar data have not been thoroughly evaluated in more general populations.3 4 Furthermore, given a low incidence of actionable items in paediatric populations, the utility of home monitoring was not as beneficial as adult populations.5 In 2016, more than 1600 CRD reports were obtained within our institution with almost 70% of reports being generated by patients from home. Prior to the improvement project, more than 30% of all device reports were either sent by patients from home or obtained in the office without prior notification of the EP service responsible for managing CRDs. This lack of notification resulted in inefficiencies in the system. Additional time was required by EP nursing and physician staff to identify why an un-notified report had been sent and who was responsible for the patient’s care and needed the report information.
Baseline measurements
Our primary measurement was the percentage of unscheduled CRD reports obtained without notification of the EP service. The rationale for this project was that lack of notification of device reporting was associated with inefficiency within the EP staff resulting in lost revenue. Additionally, there were potential safety concerns for patients secondary to the timeliness of device report processing. The project population included all patients primarily followed by a cardiologist within our institution who had CRD reports obtained within the study time period. Data on each CRD report were obtained including whether the report was scheduled or unscheduled, whether or not there was proper EP notification, where the report was generated (home vs office) and the patients managing team (EP/cardiomyopathy/adult congenital heart disease (ACHD)/General Cardiology). A scheduled CRD report was defined as a report that was scheduled in our electronic system > 7 days prior to the report generation. Logistically, electronic scheduling is typically performed by EP nursing staff directly, though can be scheduled by the general cardiology scheduling personnel. EP notification was defined as either direct phone or email contact by the patient or requesting physician with the following information: (1) Why the report was being sent, (2) What symptoms the patient was experiencing and (3) What medical staff was responsible for report findings. In addition to tracking the percentage of reports initiated without EP notification, we also tracked the average time required for report processing to evaluate changes in efficiency and workload throughout the process. The time to process a device report was determined and divided into three categories including (1) device reports that were scheduled, (2) device reports that were unscheduled but with EP notification and (3) device reports that were unscheduled and without EP notification. Time estimates for the three different categories are demonstrated in online supplementary figure S1. The time required for report processing was evaluated weekly and was calculated as follows: (average time per scheduled report)(number of reports)+(average time per unscheduled/EP notified report)(number of reports)+(time per unscheduled/EP not notified report)(number of reports)/total number of reports that week.
Supplementary file 1
Design
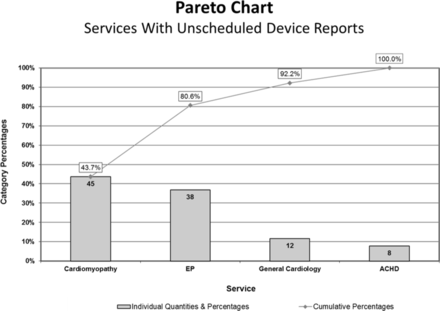
This project used the Model for Improvement. A team was created including representatives from the EP physician staff, EP nursing staff and individuals from the various cardiology subspecialties. The team analysed the process and steps required from the initiation of a CRD report to its proper reporting of results to the patient and managing physician. We identified key drivers of common factors leading to lack of EP notification. A process map was created documenting the steps required from the time of device report initiation to proper distribution of the report findings to the appropriate entities. Pareto charts determined that the EP and cardiomyopathy services were key cardiac subspecialties (figure 1) and device reporting initiated by patients from home (data not shown) as common causes for unscheduled reporting. Our failure mode and effects analysis (FMEA) map demonstrated that common failures in EP notification were driven by the lack of an easy to use method to notify the EP service and a lack of patient and physician understanding about the notification process and the importance of EP notification.
Pareto chart of cardiology service associated with unscheduled reports. Chart of the percentage of patients having unscheduled device reports according to the primary managing cardiology service. ACHD, adult congenital heart disease service; EP, electrophysiology service.
Strategy
We initially created a SMART Aim to decrease the percentage of device reports without EP notification from 30% to 20% by April 2017. Secondary to early success, that AIM was modified to 10% midway through the project.
PDSA cycle 1 involved the creation of a universal and easy to use EP notification system. A common telephone number and email address was created specifically for the use of report notification. The phone line and email was accessible to both patients and families and was monitored by all of the EP nursing staff. This intervention was associated with a decrease in CRD reports without EP notification from 30% to 21%.
PDSA cycle 2 involved education of the various cardiology subspecialties taking care of patients with CRDs. This PDSA cycle was approached in a Ramp fashion targeting the differing subspecialty services independently. For each subspecialty service, a group was formed including one or more physicians and nurses from that subspecialty service. After discussion around ongoing issues around device notification and proper notification protocols in that small group, a presentation was made to the subspecialty service as a whole. This Ramp began with the EP service and was subsequently rolled out to the cardiomyopathy, ACHD and general cardiology services sequentially. In total, this intervention improved the percentage of EP reports with notification from 21% to 10%.
Next we performed several interventions targeted at patient education including PDSA cycles 3–5. PDSA cycle 3 involved patient education with the use of our follow-up reminder cards. Following each visit, patients had historically been given a wallet sized reminder card with information about their next follow-up visit. In this intervention, information with regard to proper home CRD reporting including proper notification was placed on the back of this follow-up card. At the end of each visit, the patient was given this follow-up card and the EP nursing staff reviewed the notification process with the patient and their family.
PDSA cycle 4 involved EP clinic coordinator patient phone call for any CRD report obtained without EP notification. We identified that patients who had not yet circulated through the office for their routine follow-up visits were unaware of the new reporting notification process. Additionally, some patients required additional teaching. In this intervention, all patients who sent in CRD reports without proper EP notification were contacted by the EP clinic co-ordinator to identify if there was an ongoing medical issue with patient and additional teaching was provided with regards to proper report notification.
PDSA cycle 5 involved patient education via an informational letter sent to patient’s home. A letter containing the proper notification process was sent to the homes of all patients followed at our institution with a CRD. The effectiveness of the home letter was questionable, and several patients called or sent in a device transmission in response to receiving the letter. Each of these patients was contacted and given verbal information with regard to the new notification process.
In total, the patient education interventions were responsible for a decrease in CRD reporting without notification from 10% to 5%.
Results
The primary measure of this project was the percentage of CRD reports obtained without EP notification. Over the study time period, each CRD report was evaluated and a database was created tracking whether or not the report was unscheduled as well as whether there was EP notification. Pareto and FMEA charts determined that failures were commonly related to lack of an easy to use EP notification system and poor patient and/or physician understanding of CRD reporting process. Furthermore, specific services including the EP service and cardiomyopathy services, as well as reports generated by patients from home, were responsible for the majority of failures. We performed multiple interventions including (1) creation of a single, easy to use method of EP notification that could be used by patients and medical staff; (2) physician education; (3) patient education; and (4) linkage of device reporting protocol with pre-existing follow-up cards.
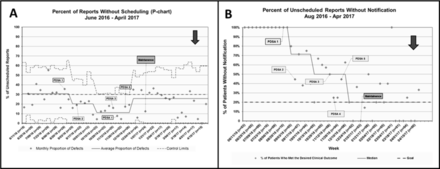
When the project started, just over 30% of all CRD reports were obtained without notification of the EP service. Following our project interventions, only 5% of CRD reports were obtained without notification (figure 2). The response to the individual improvements was described in the strategy section for each PDSA cycle. To further elucidate the underlying mechanisms for this improvement, we also tracked the percentage of patients with unscheduled reports which was highly associated with lack of EP notification as well as the percentage of unscheduled reports that lacked EP notification. During the project, there was an initial improvement with a decrease in the unscheduled reports from 30% to 11% though this change was not sustained and rose to 28% by the end of the project (figure 3A). While there was not a sustained improvement in unscheduled reports, there was a sustained improvement in the percentage of unscheduled reports with proper EP notification (figure 3B) indicating that the overall improvement seen with this project was driven by improved notification rather than a decrease in overall unscheduled reporting.
Percent of device reports with EP notification. EP, electrophysiology.
Percentages of cardiac rhythm device reports (A) without scheduling and (B) unscheduled reports without electrophysiology notification.
This improvement in CRD report EP notification was associated with concomitant improvement in EP staff efficiency evidenced by a decrease in the average time for CRD report processing. At the beginning of the project, the average time for a CRD report to be processed was 38 min (figure 4). By the end of the project, the average time to process a CRD report dropped to 25 min representing a 34% reduction.
Changes in the average time of device report processing.
Lessons and limitations
CRD device reporting is a complex system which requires cooperation and coordination from multiple entities including patients, cardiologists, EP nursing and non-EP physicians. While advances in device reporting technology have improved the ease in which device reports can be obtained, this improvement has also lead to inefficiencies and potential risks secondary to poor communications about why these reports were being obtained. We found that the primary drivers of failure of patients and physicians to properly notify the EP service of a device transmission stemmed from a poor understanding of the reporting process and the downstream effects of poor communication. Additionally, patients and physicians are busy and require an easy to use, unified system for notification in the event of an urgent unscheduled CRD transmission. Through the creation of an easy to use notification system linked with patient and physician education, we were able to significantly improve the percentage of reports with proper EP notification. This improvement was additionally linked to improved work efficiency for EP nurses and staff.
We felt there were certain limitations in what interventions we chose to use. Specifically, we did want to enact interventions which might affect patient non-compliance with routine device reporting. We specifically chose not to use punitive measures at the patient or physician level and avoided information that would create a negative perspective of device reporting in general. Additionally, we found that patient distributed information in the form of mailers without verbal reinforcement was less useful. In fact, this intervention may even result in increased reporting without notification and direct patient contact with EP staff was required for effective patient education. Lastly, we did not directly involve a member of our team specifically from business or nursing administration which may have aided further analysis of downstream effects of cost or time analysis.
Conclusions
Utilisation of quality improvement methodology led to a significant improvement in EP notification of CRD device reporting as well as improved understanding and cooperation from both patients and medical staff in CRD reporting process. This systematic improvement was associated with improved efficiency and reduction in nursing time required for report processing.
Footnotes
Contributors All authors directly contributed to significant aspects of study design, implementation, statistical analysis and manuscript creation/revision.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data sharing not available.